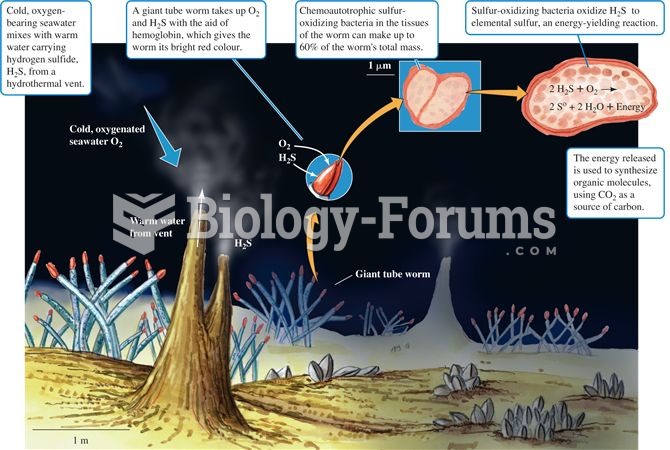
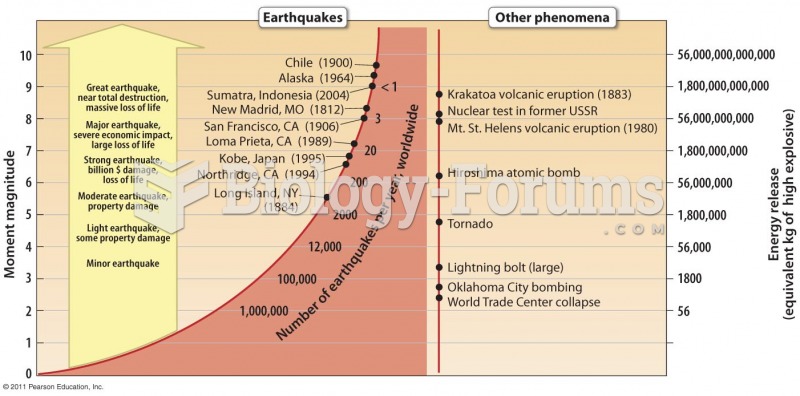
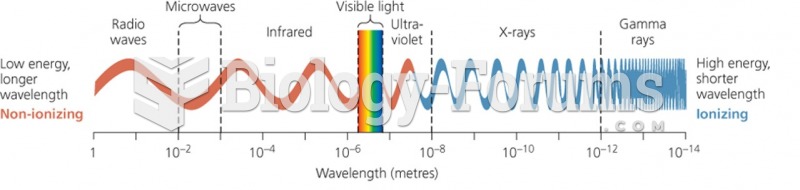
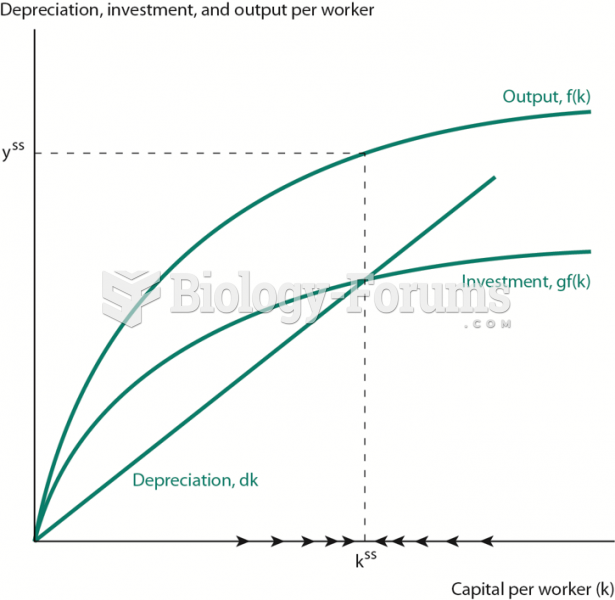
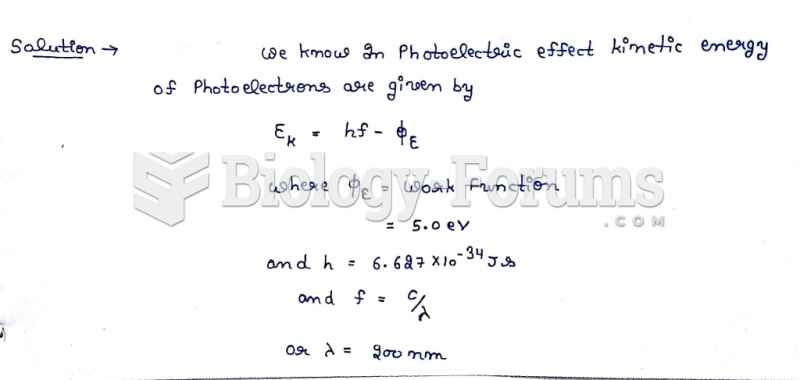
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.