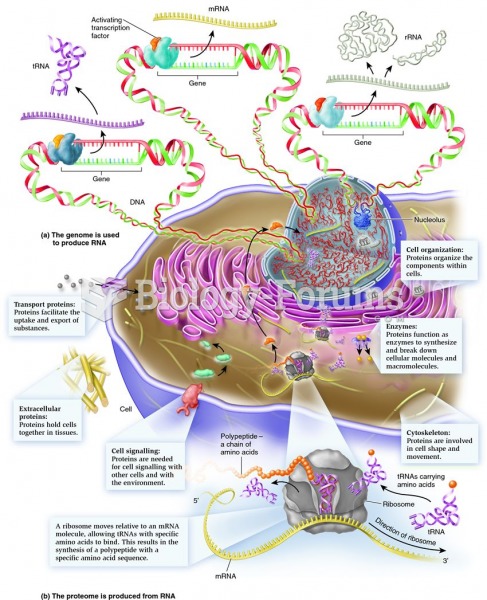
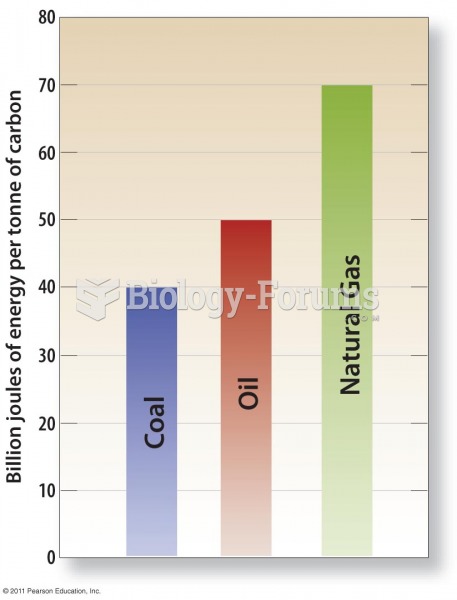
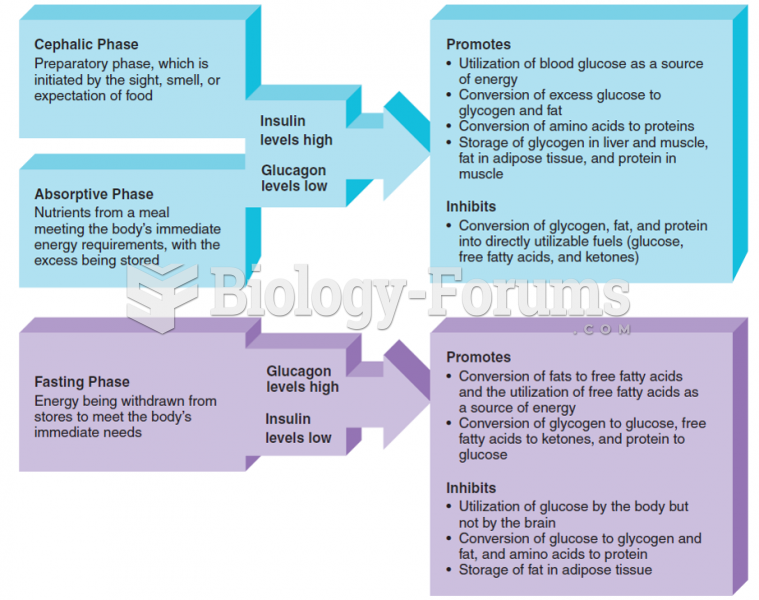
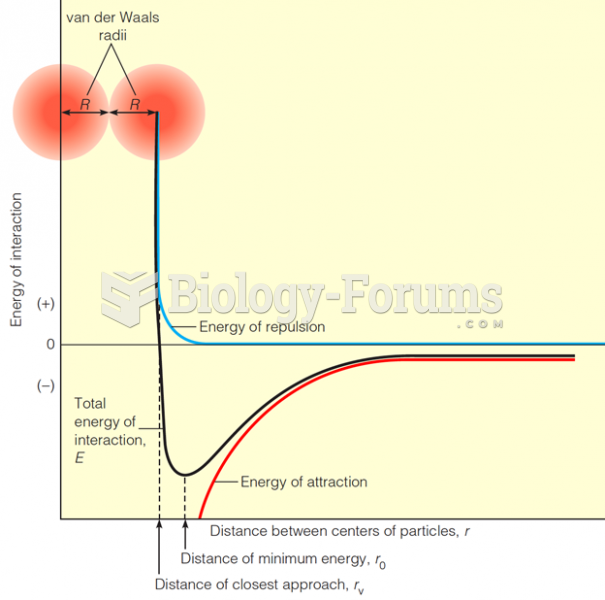
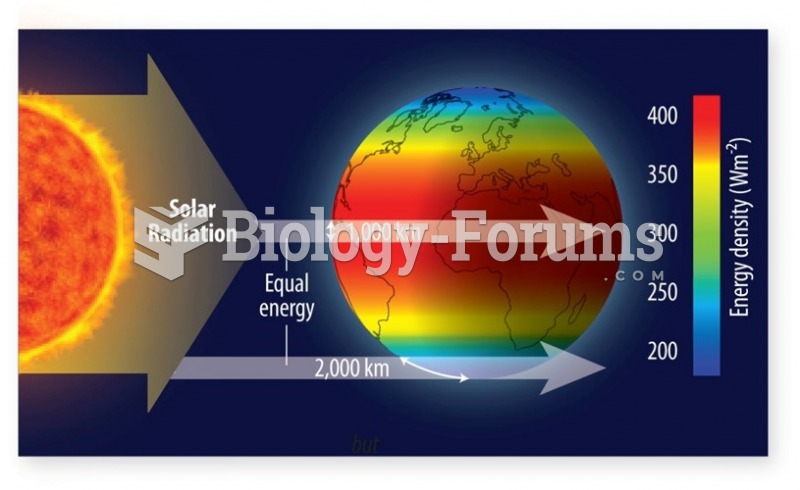
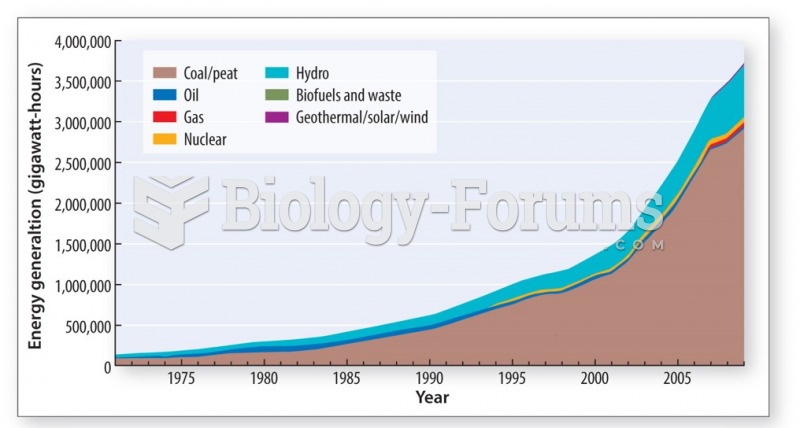
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Did you know?
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.
Did you know?
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.