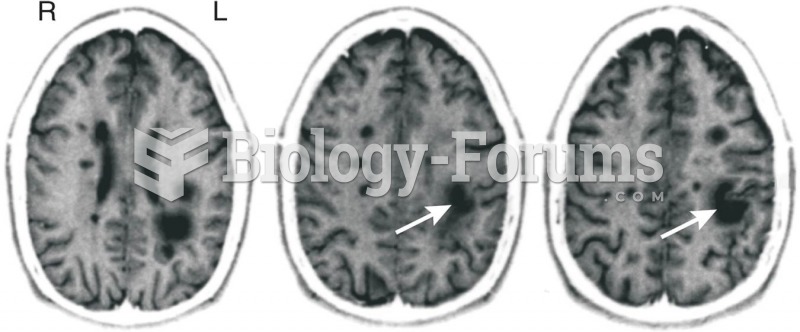
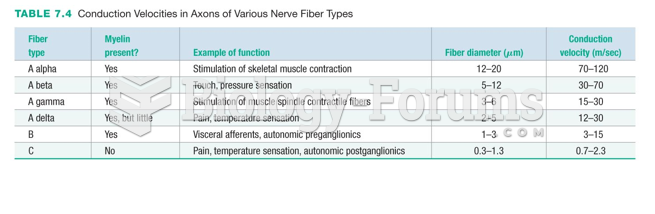
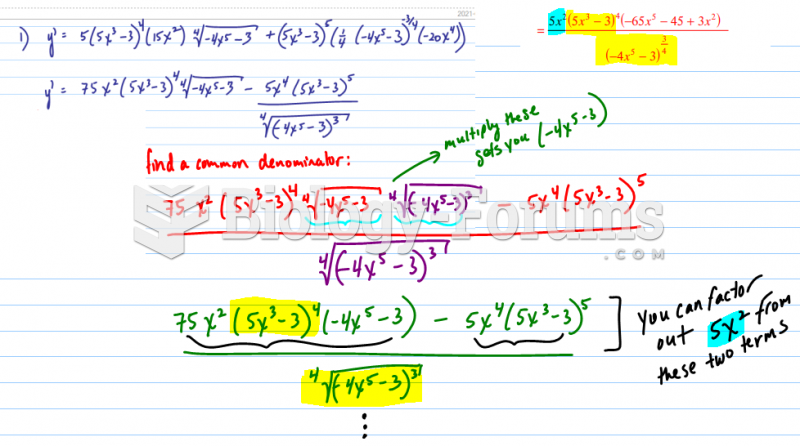
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.