This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
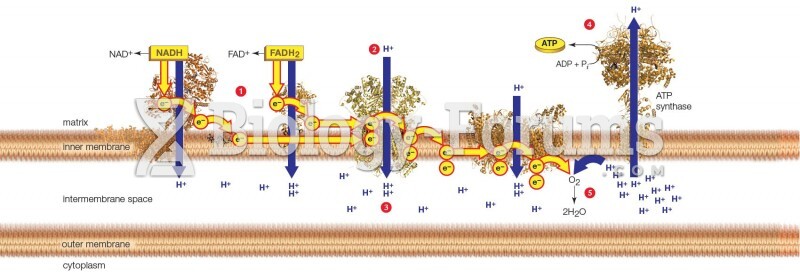
Cyanide works by making the human body unable to use oxygen.
Did you know?
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.