|
|
|
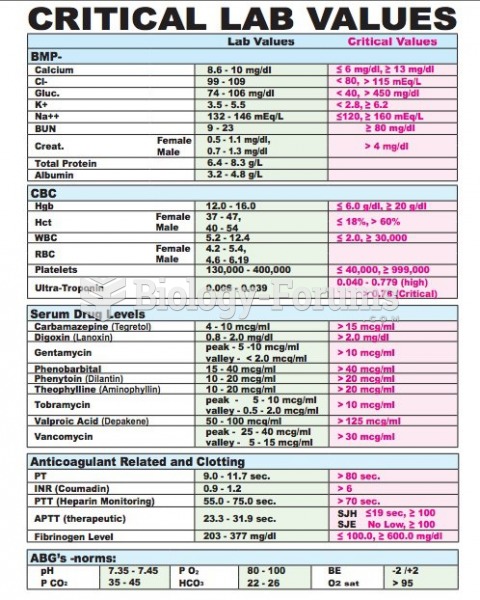
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
There are 60,000 miles of blood vessels in every adult human.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.




![Given the values of sin θ, determine the values of θ over the interval [0, 2pi]](https://biology-forums.com/gallery/42/medium_6_04_01_21_12_54_15.png)
