|
|
|
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
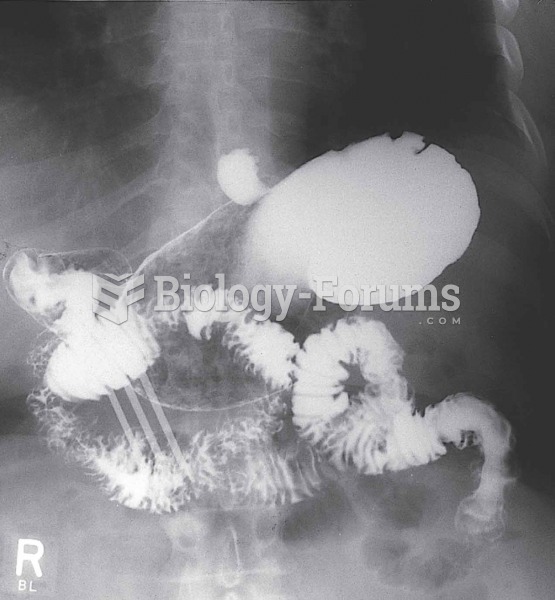
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.