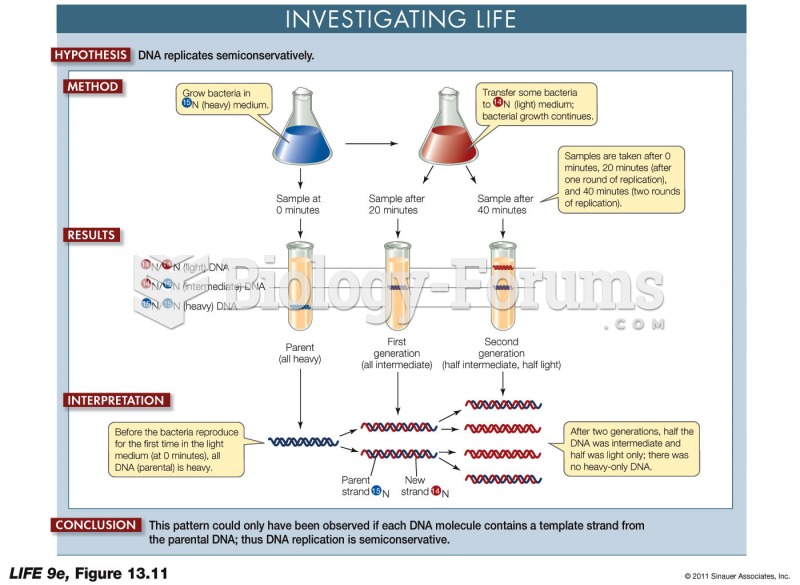
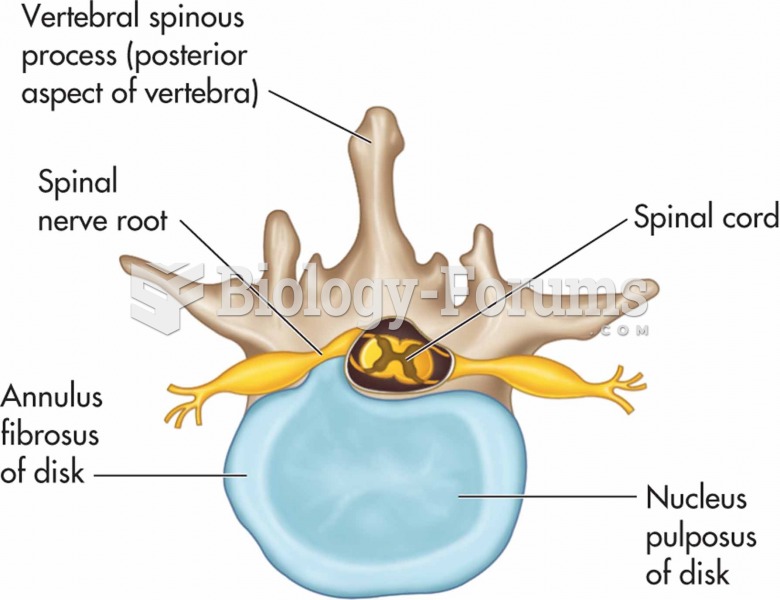
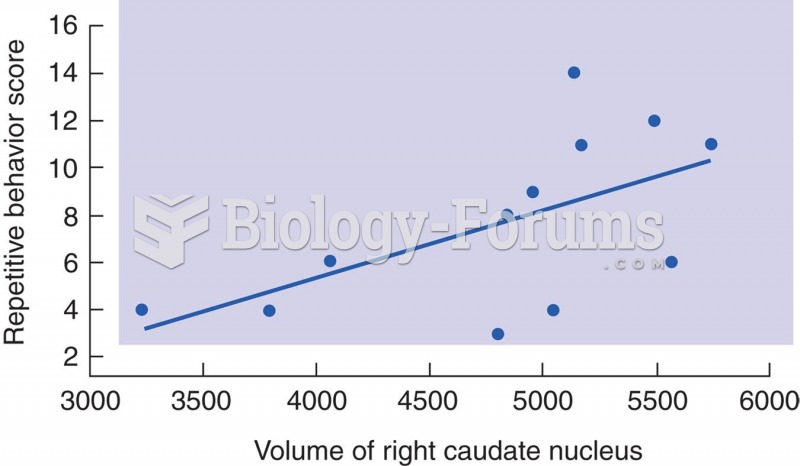
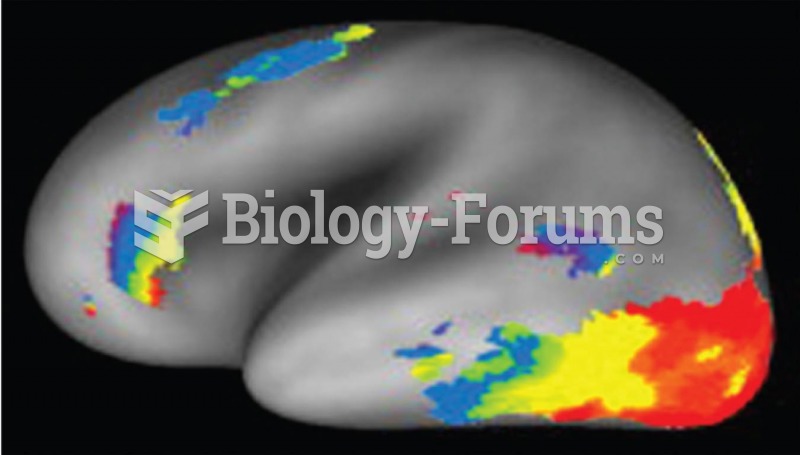
|
|
|
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.
Certain topical medications such as clotrimazole and betamethasone are not approved for use in children younger than 12 years of age. They must be used very cautiously, as directed by a doctor, to treat any child. Children have a much greater response to topical steroid medications.