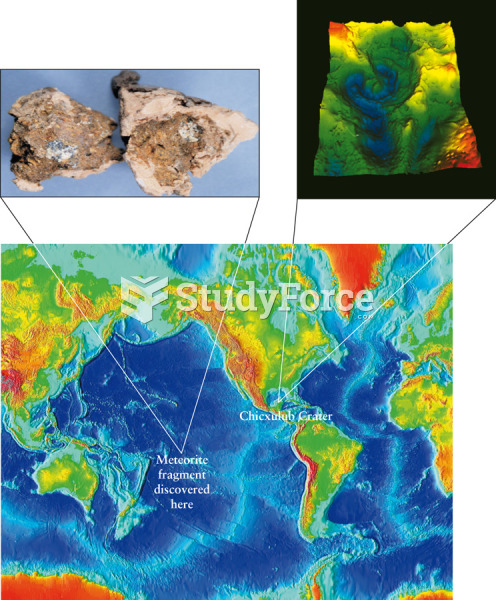
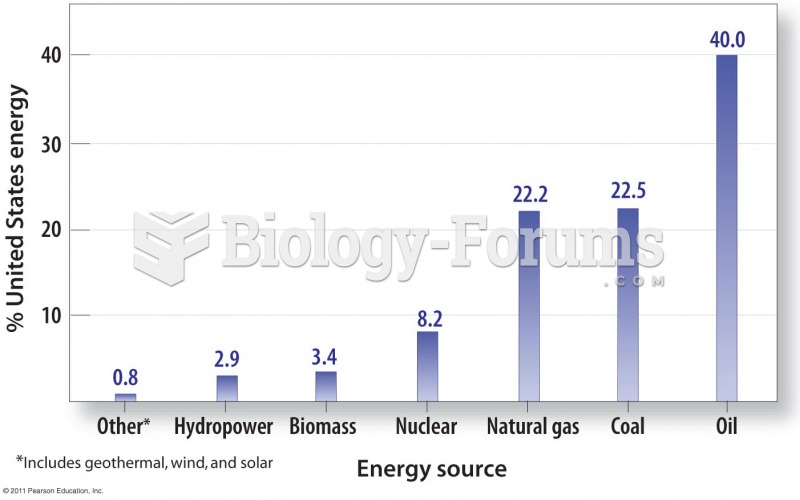
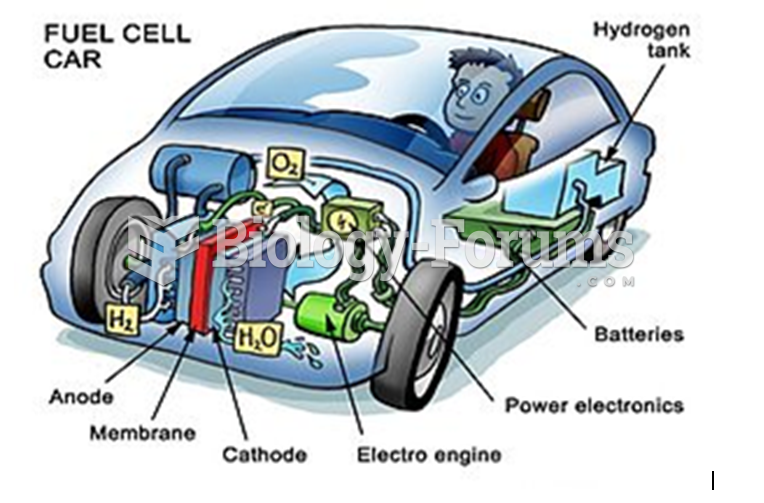
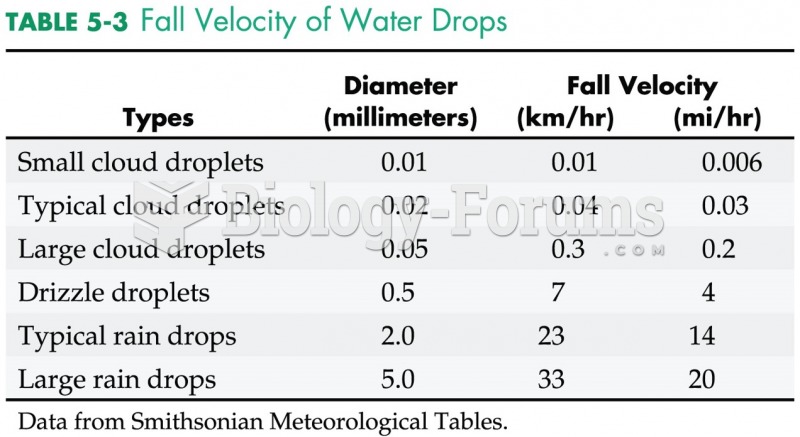
|
|
|
Medication errors are more common among seriously ill patients than with those with minor conditions.
Oxytocin is recommended only for pregnancies that have a medical reason for inducing labor (such as eclampsia) and is not recommended for elective procedures or for making the birthing process more convenient.
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.