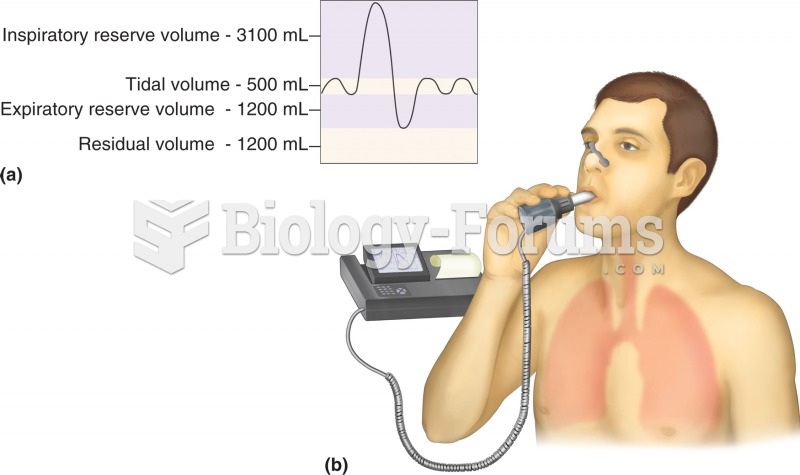
|
|
|
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
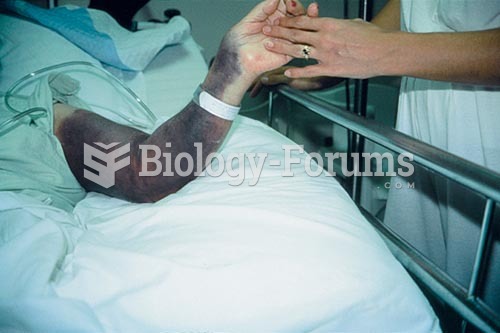 Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
 Sphygmomanometry. Photograph of a physician taking blood pressure readings with the use of a sphygmo
Sphygmomanometry. Photograph of a physician taking blood pressure readings with the use of a sphygmo