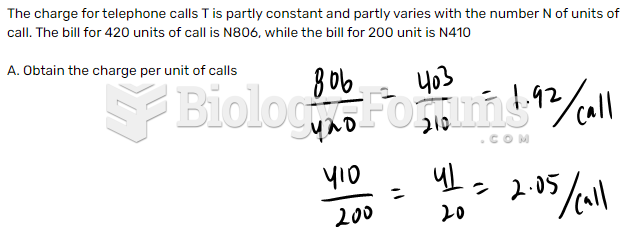This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Did you know?
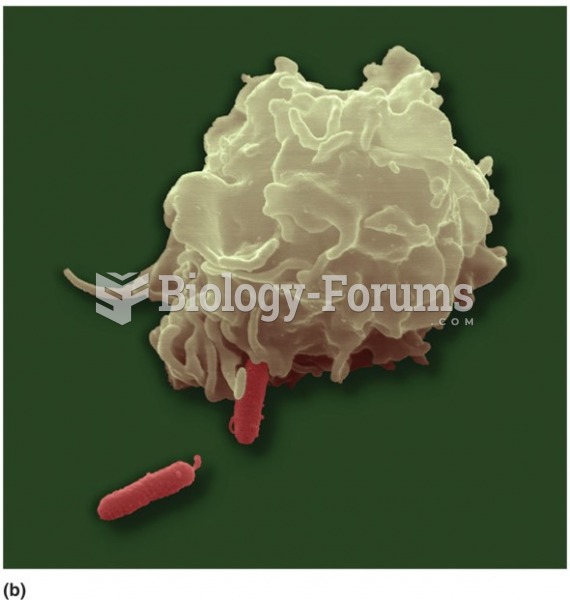
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
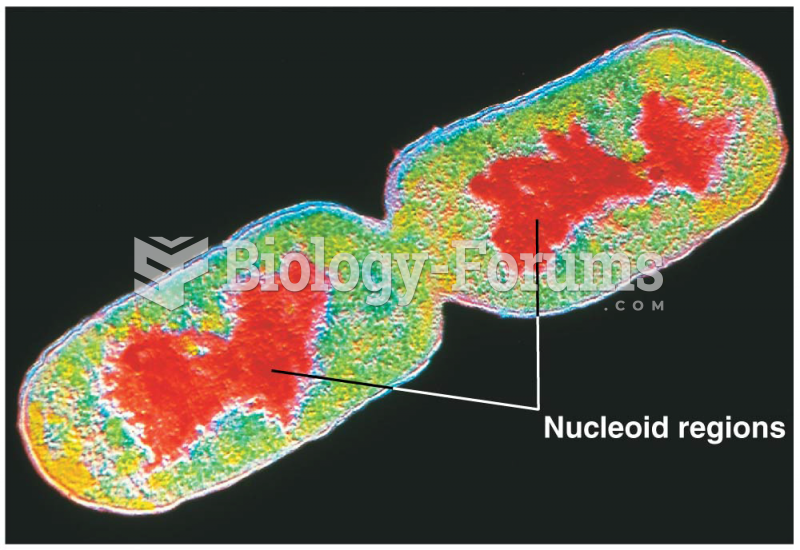
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.