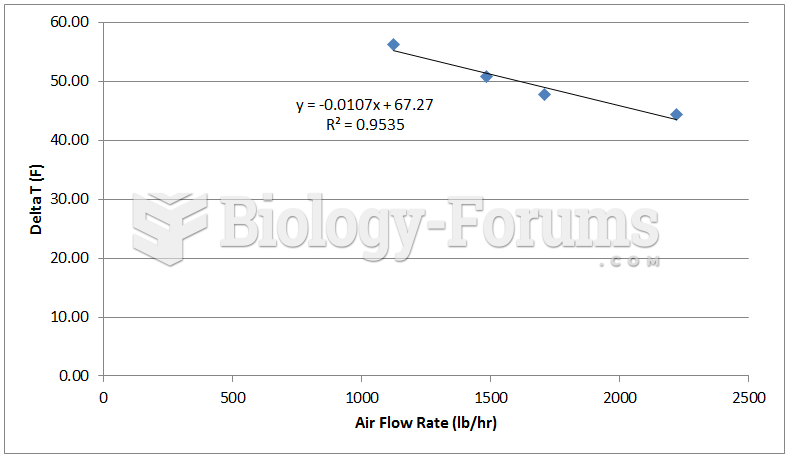
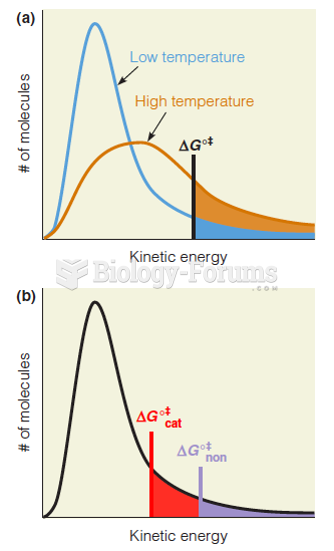
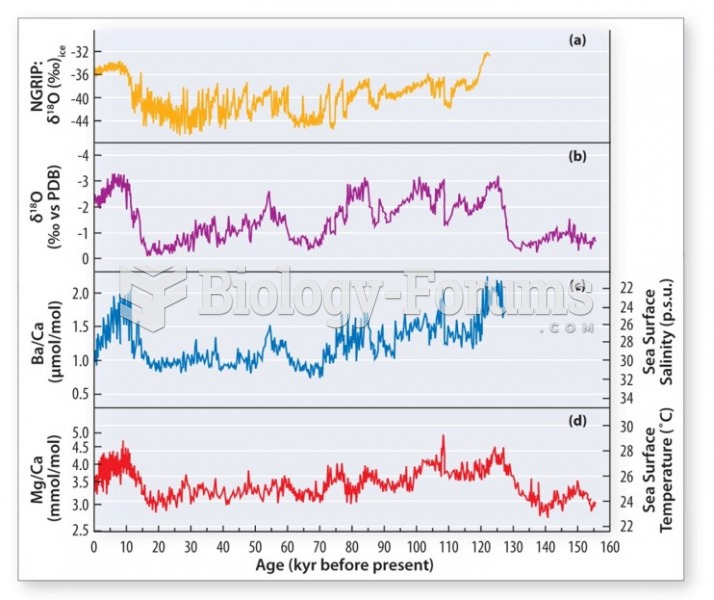
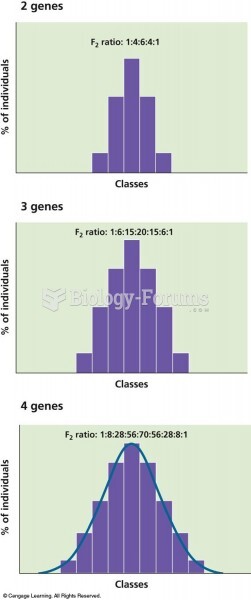
|
|
|
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.