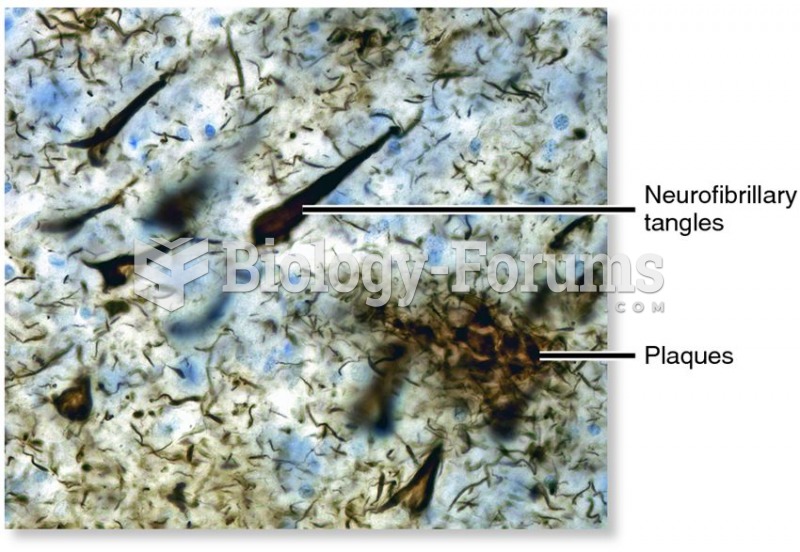
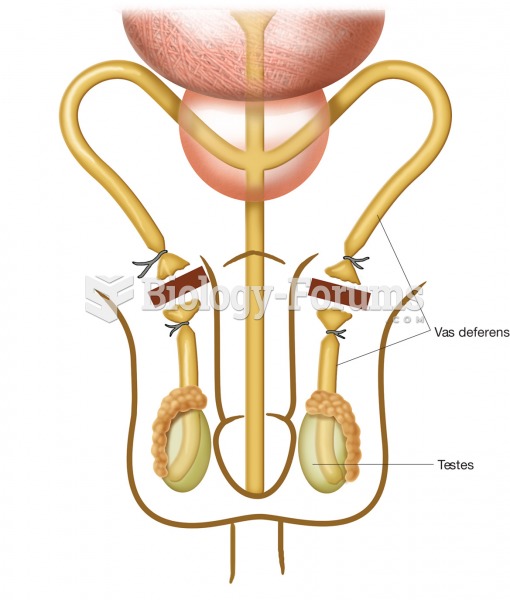
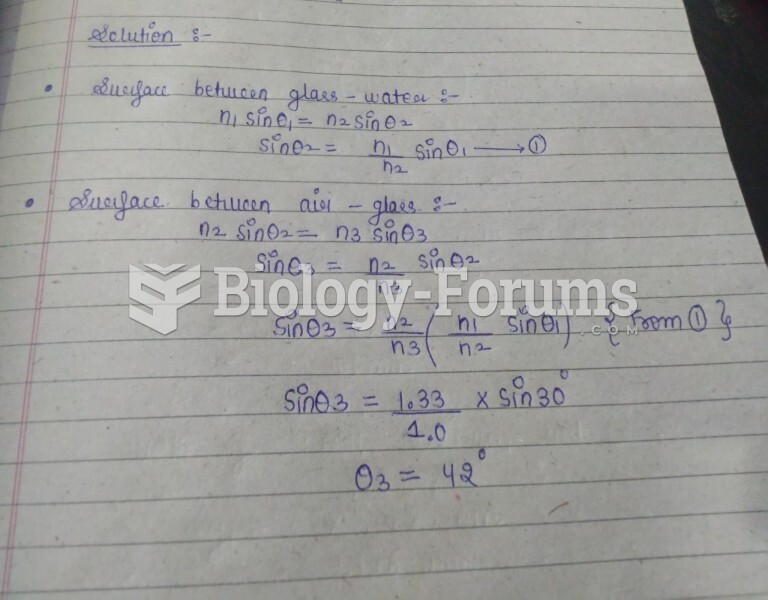
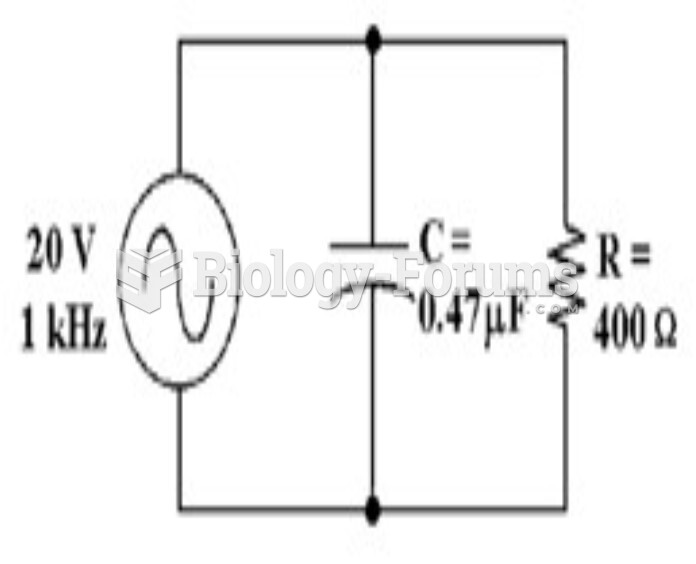
|
|
|
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Asthma-like symptoms were first recorded about 3,500 years ago in Egypt. The first manuscript specifically written about asthma was in the year 1190, describing a condition characterized by sudden breathlessness. The treatments listed in this manuscript include chicken soup, herbs, and sexual abstinence.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
There are 20 feet of blood vessels in each square inch of human skin.
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.