|
|
|
Every flu season is different, and even healthy people can get extremely sick from the flu, as well as spread it to others. The flu season can begin as early as October and last as late as May. Every person over six months of age should get an annual flu vaccine. The vaccine cannot cause you to get influenza, but in some seasons, may not be completely able to prevent you from acquiring influenza due to changes in causative viruses. The viruses in the flu shot are killed—there is no way they can give you the flu. Minor side effects include soreness, redness, or swelling where the shot was given. It is possible to develop a slight fever, and body aches, but these are simply signs that the body is responding to the vaccine and making itself ready to fight off the influenza virus should you come in contact with it.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Vaccines prevent between 2.5 and 4 million deaths every year.
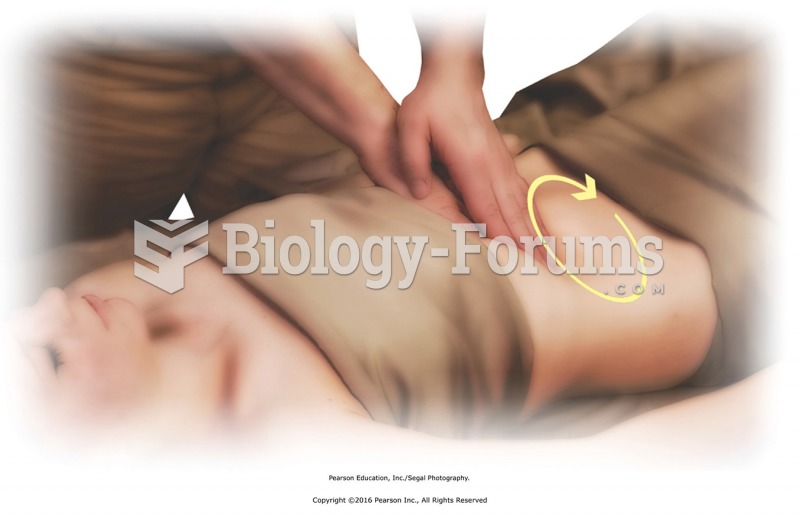 Effleurage clockwise to abdomen. Place palm over palm, and apply effleurage in a circle around the ...
Effleurage clockwise to abdomen. Place palm over palm, and apply effleurage in a circle around the ...
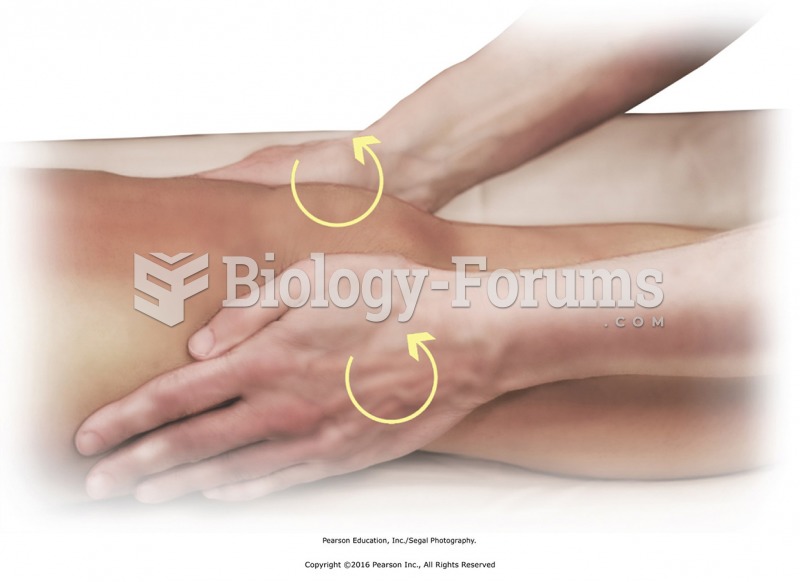 Apply circular friction with the heels of the hands to tendons that cross the knee. Place heels of ...
Apply circular friction with the heels of the hands to tendons that cross the knee. Place heels of ...