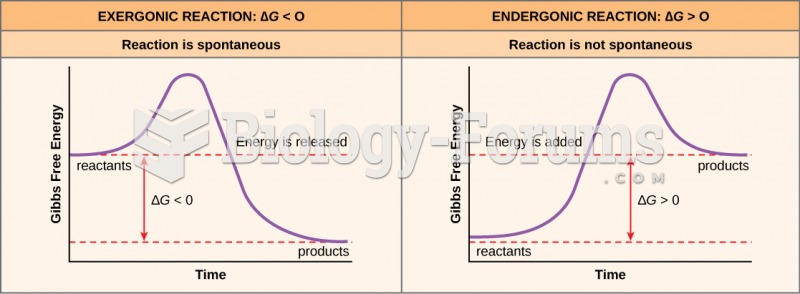
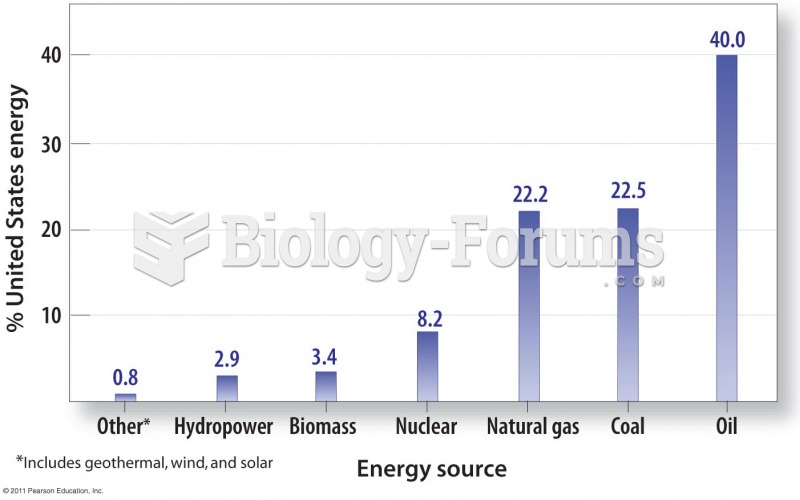
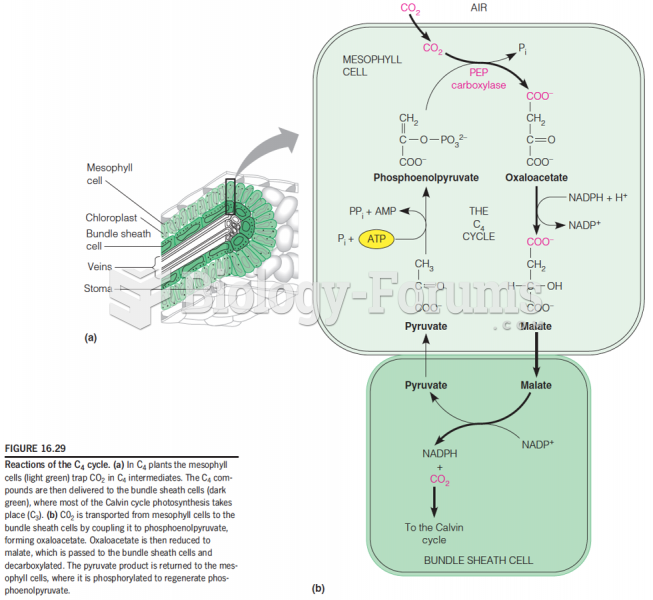
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.