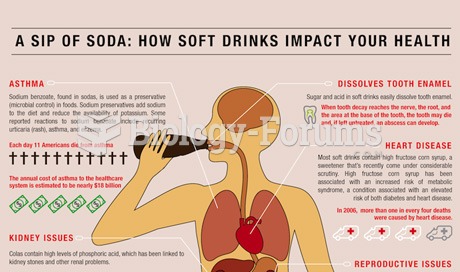
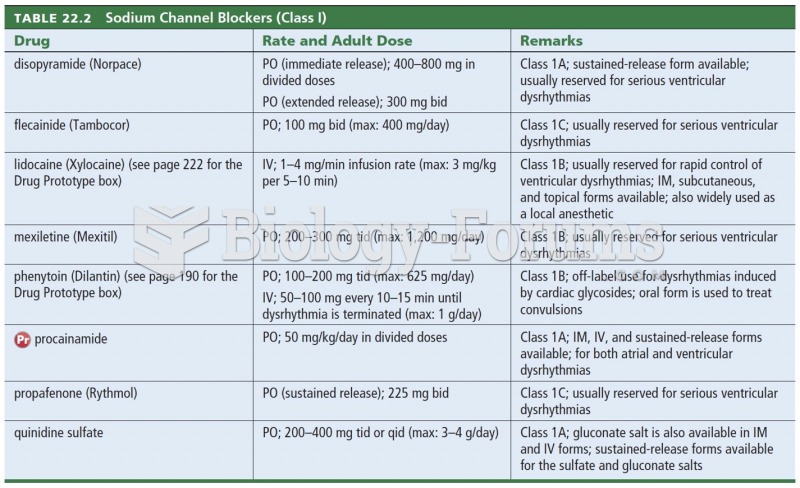
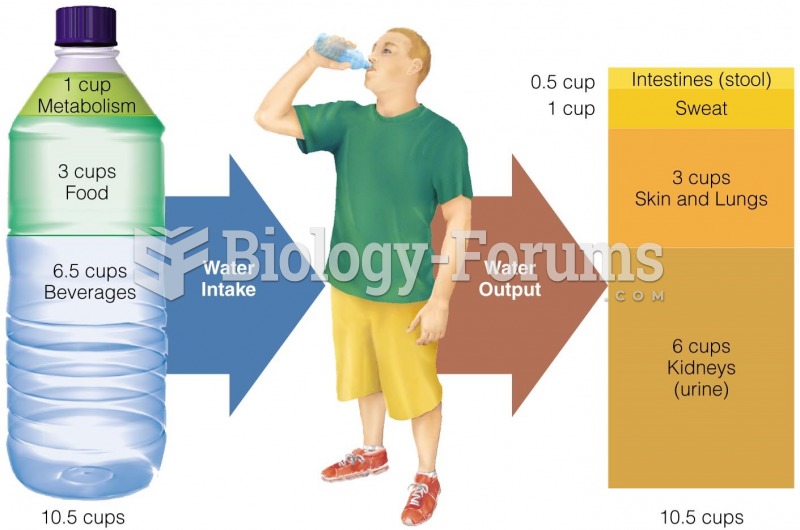
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Did you know?
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.