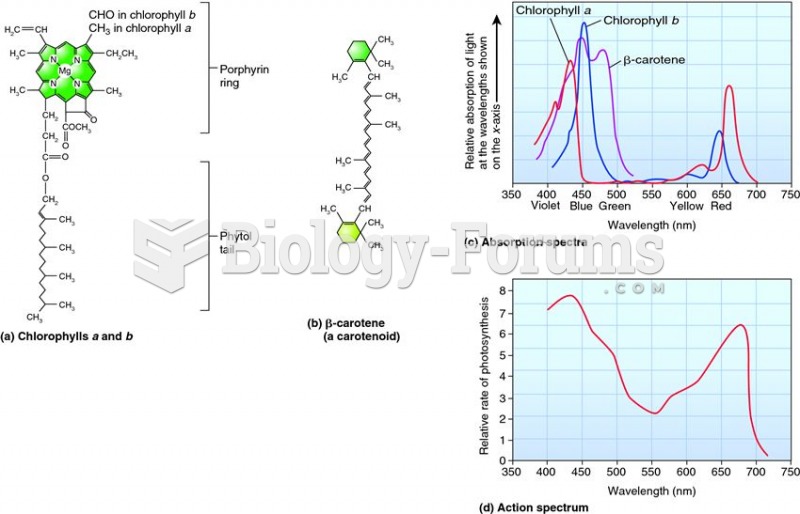
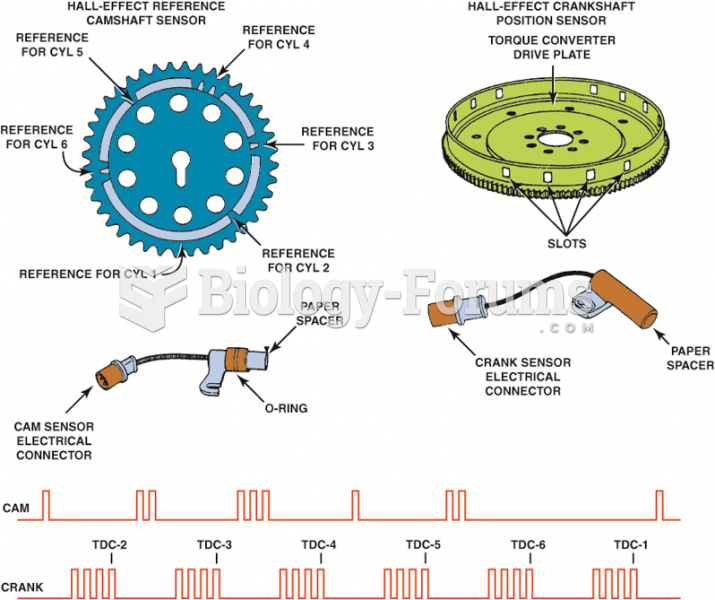
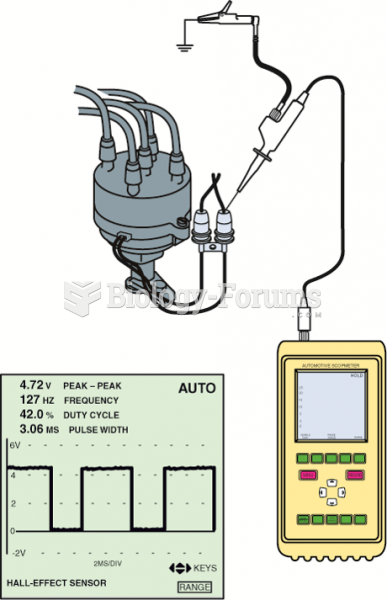
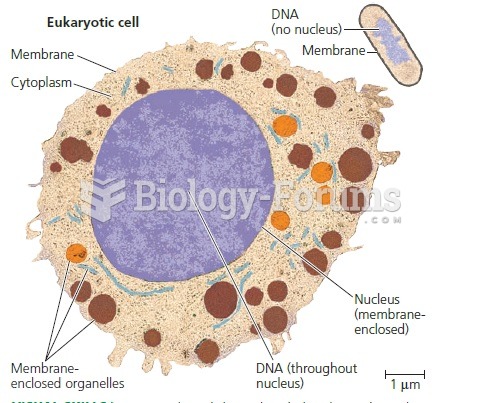
|
|
|
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Vaccines cause herd immunity. If the majority of people in a community have been vaccinated against a disease, an unvaccinated person is less likely to get the disease since others are less likely to become sick from it and spread the disease.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.