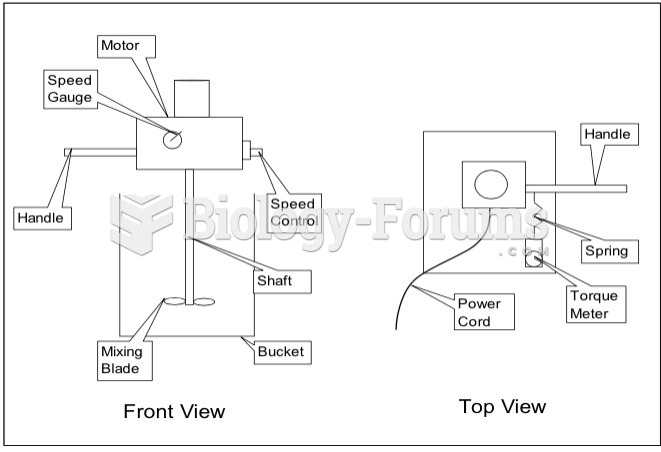
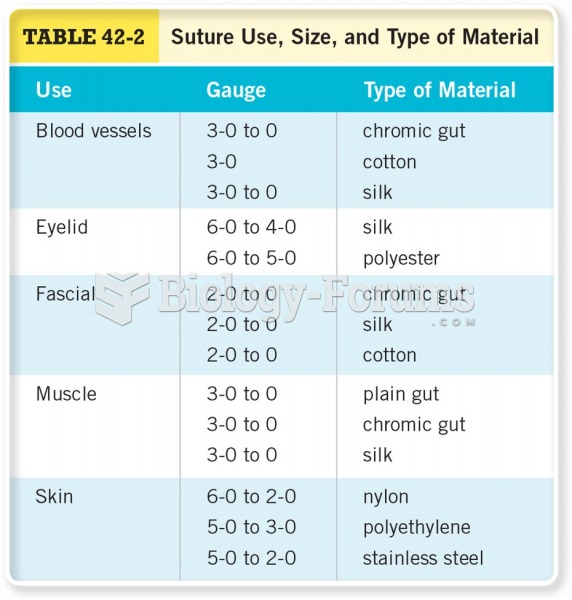
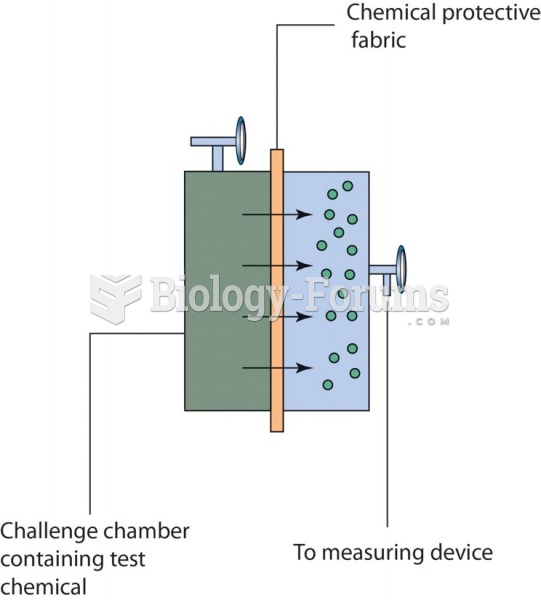
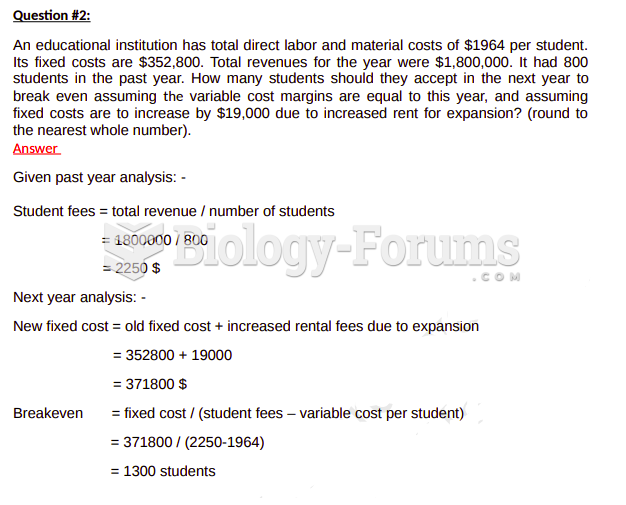
|
|
|
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
 Measure between terminal 30 and 87a. Terminal 87a is the normally closed contact, and there should ...
Measure between terminal 30 and 87a. Terminal 87a is the normally closed contact, and there should ...
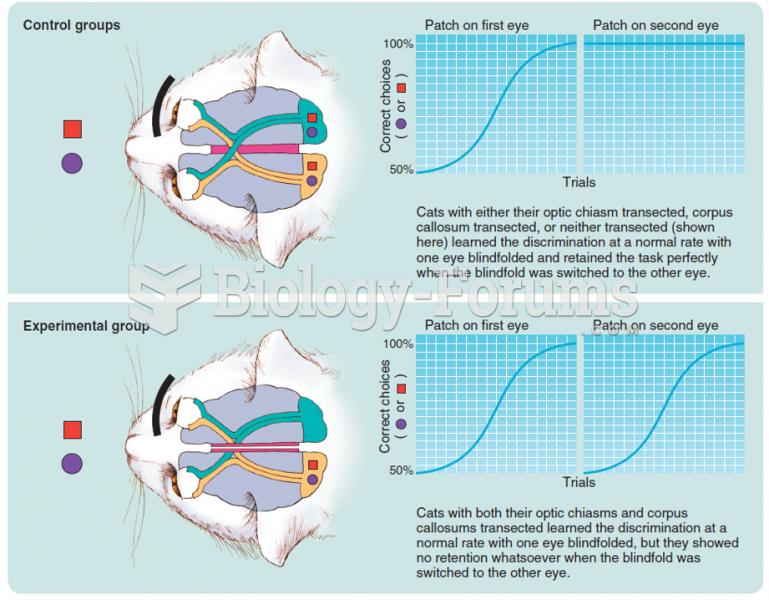 Schematic illustration of Myers and Sperry’s (1953) groundbreaking split-brain experiment. There ...
Schematic illustration of Myers and Sperry’s (1953) groundbreaking split-brain experiment. There ...