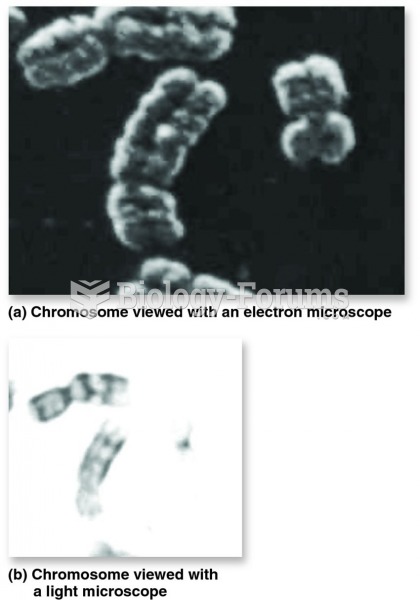
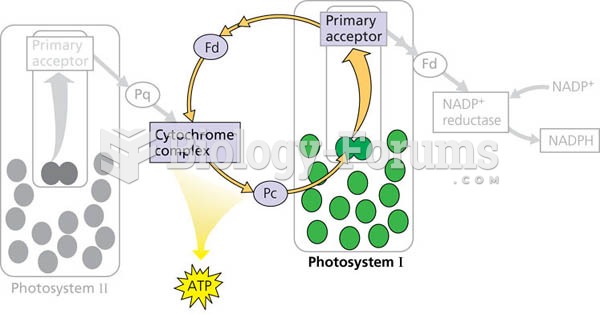
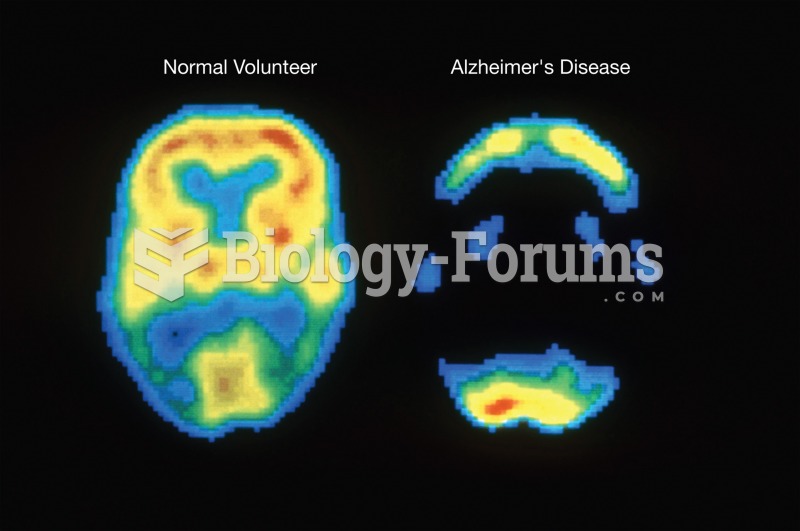
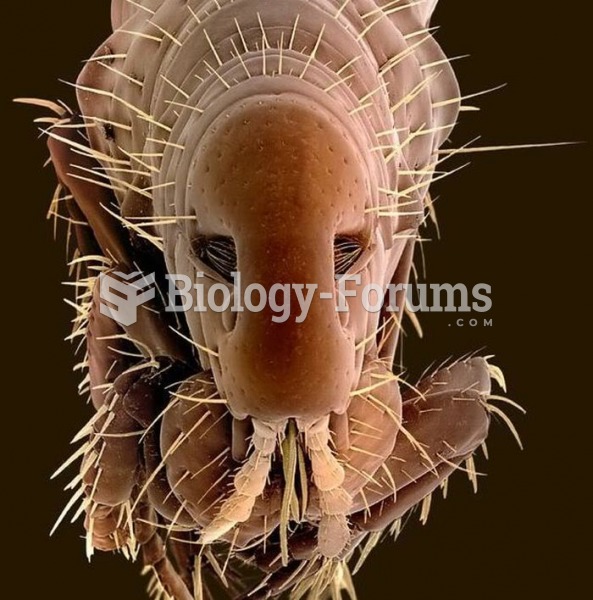
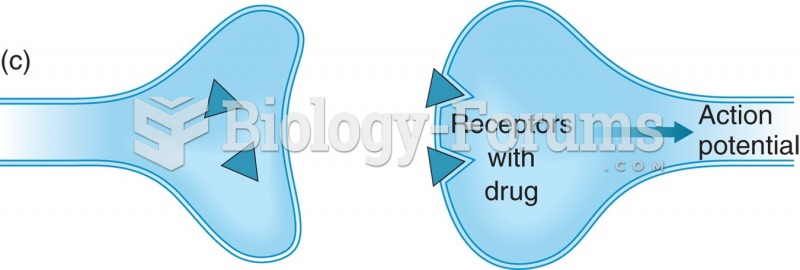
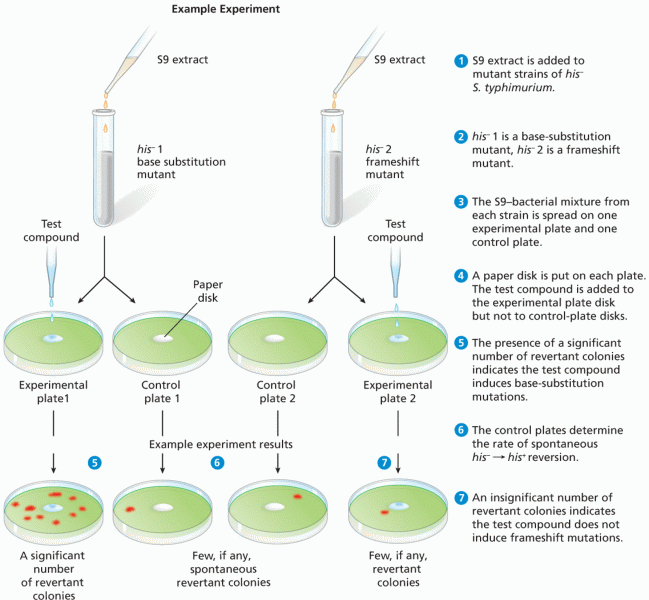
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Never take aspirin without food because it is likely to irritate your stomach. Never give aspirin to children under age 12. Overdoses of aspirin have the potential to cause deafness.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.