|
|
|
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
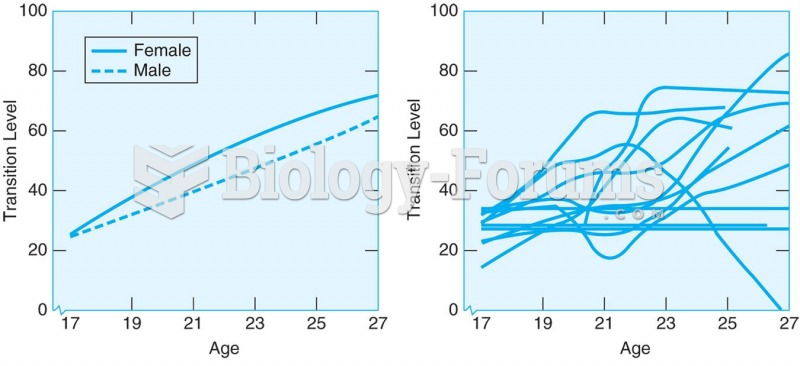 (Left panel) The transition level (TL) for moving out of the parental home for 240 young men and ...
(Left panel) The transition level (TL) for moving out of the parental home for 240 young men and ...
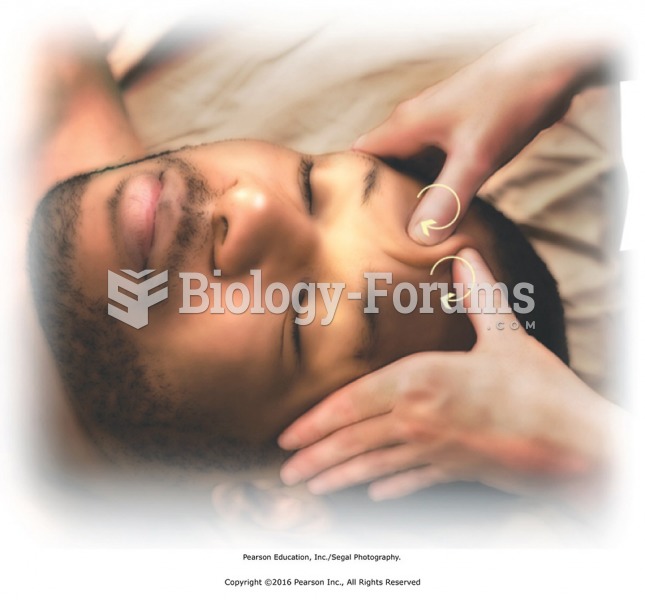
 Lightly squeeze along each finger applying pressure first near the knuckle and then moving along the ...
Lightly squeeze along each finger applying pressure first near the knuckle and then moving along the ...