This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The term pharmacology is derived from the Greek words pharmakon("claim, medicine, poison, or remedy") and logos ("study").
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
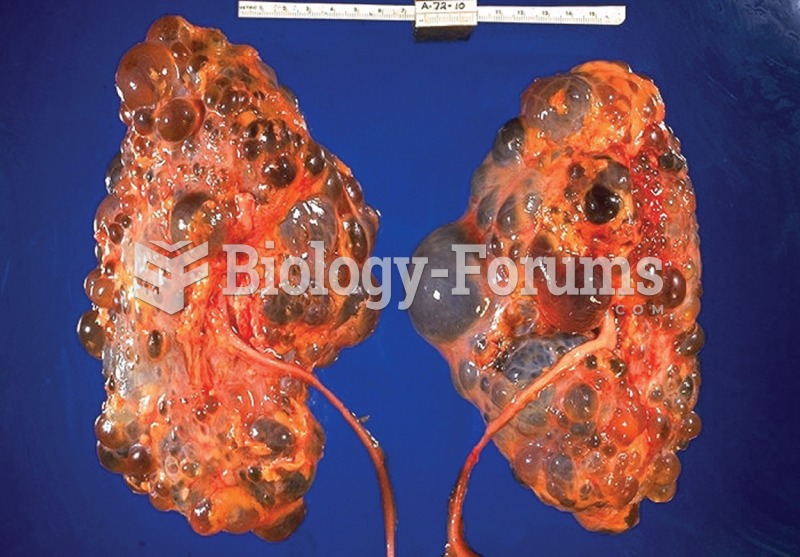
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.