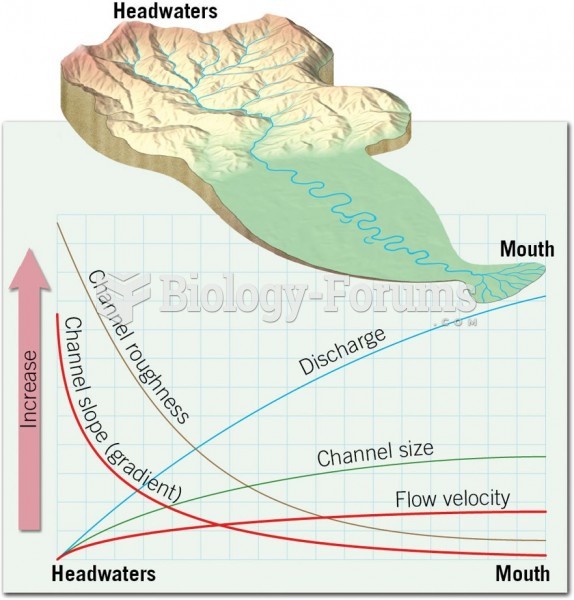
|
|
|
Although the Roman numeral for the number 4 has always been taught to have been "IV," according to historians, the ancient Romans probably used "IIII" most of the time. This is partially backed up by the fact that early grandfather clocks displayed IIII for the number 4 instead of IV. Early clockmakers apparently thought that the IIII balanced out the VIII (used for the number 8) on the clock face and that it just looked better.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
If you could remove all of your skin, it would weigh up to 5 pounds.
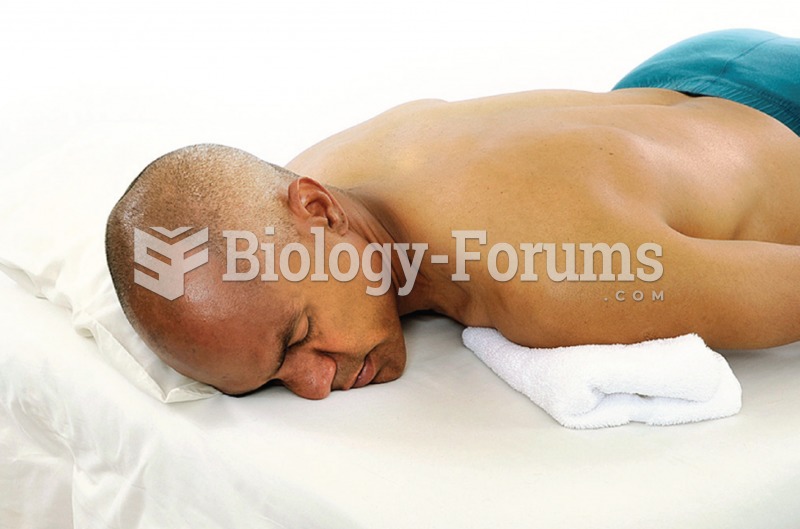 To reduce stress on your client’s neck when the head is turned, put a towel under the shoulder and ...
To reduce stress on your client’s neck when the head is turned, put a towel under the shoulder and ...
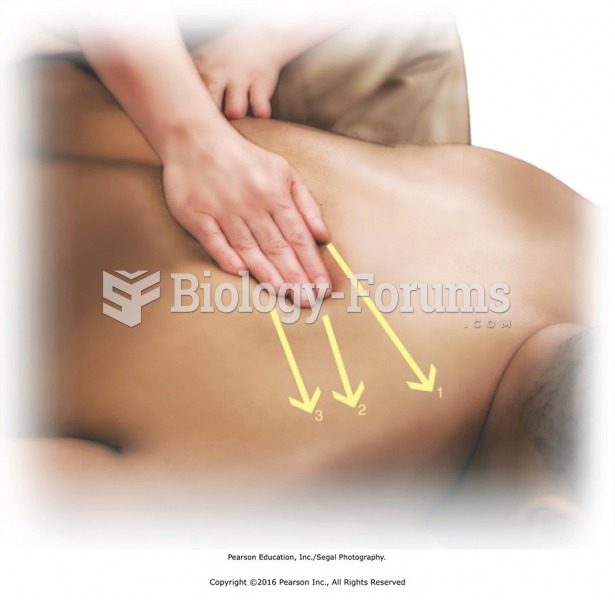 Walk around the head of the table to the left side and reach across to perform threecount stroking ...
Walk around the head of the table to the left side and reach across to perform threecount stroking ...