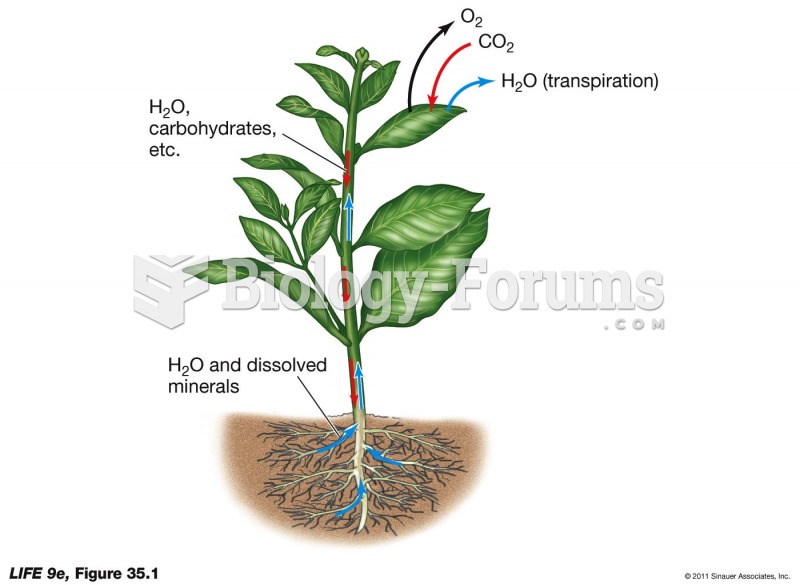
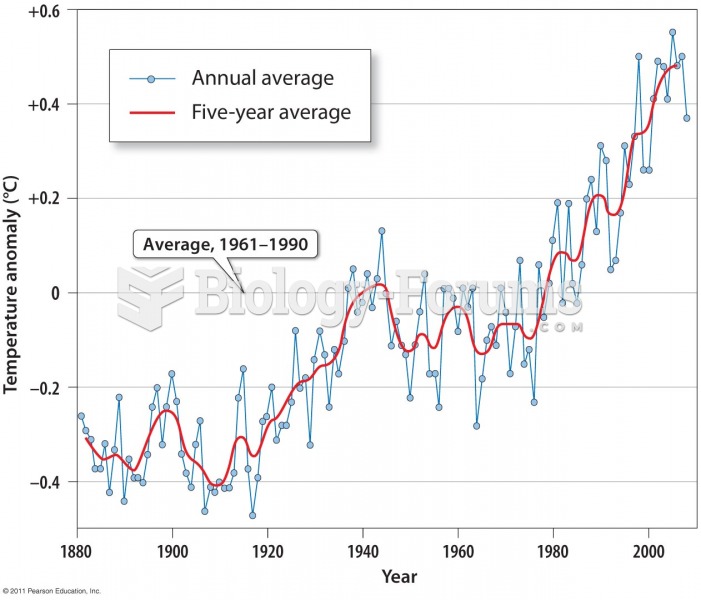
|
|
|
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.