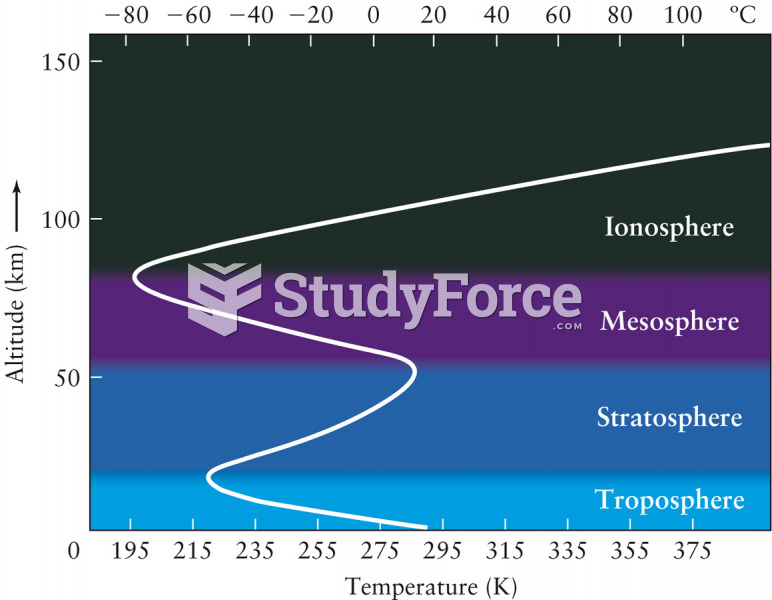
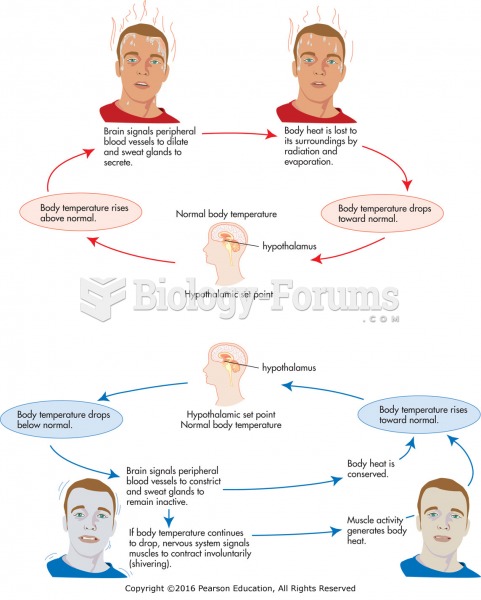
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
Did you know?
Drugs are in development that may cure asthma and hay fever once and for all. They target leukotrienes, which are known to cause tightening of the air passages in the lungs and increase mucus productions in nasal passages.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.