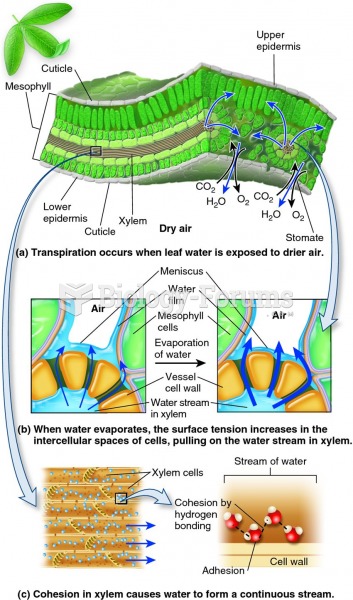
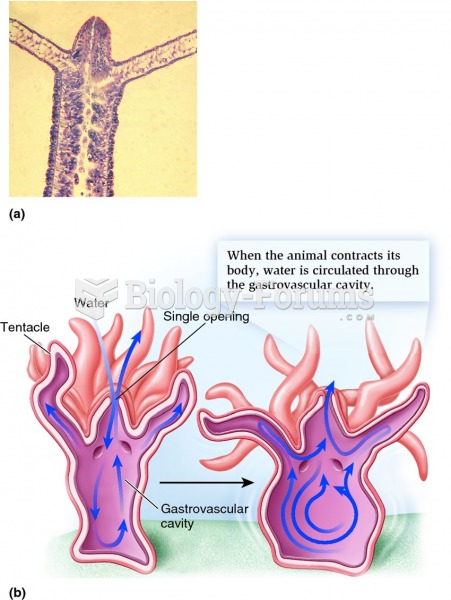
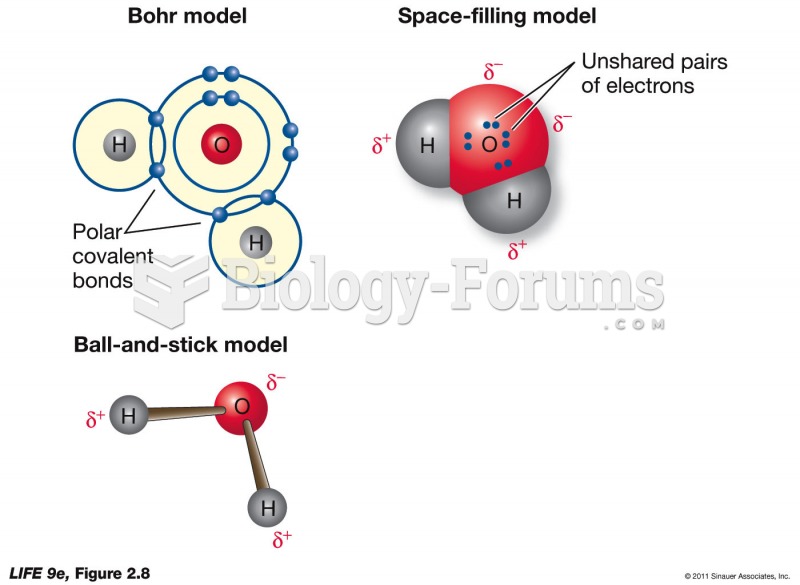
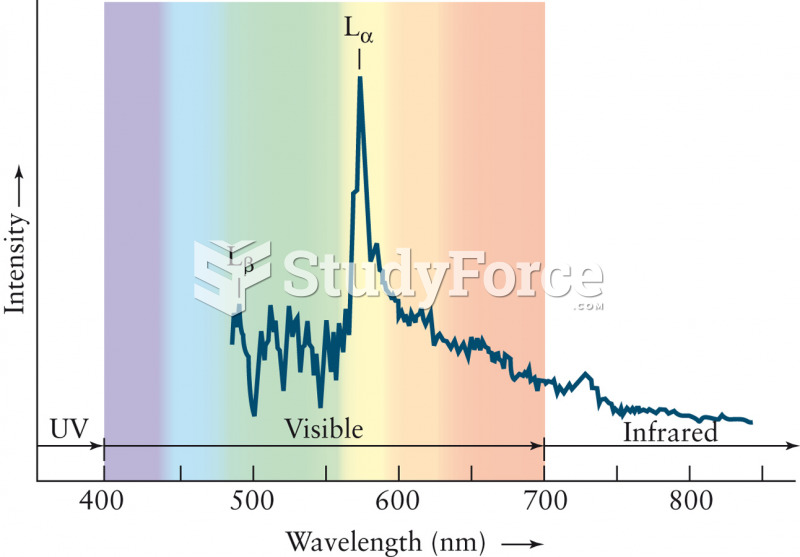
|
|
|
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.