|
|
|
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
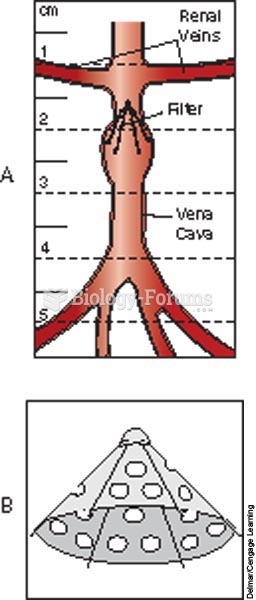 Filter in the vena cava prevents an embolus from traveling to the heart, lungs, or brain; A, Greenfi
Filter in the vena cava prevents an embolus from traveling to the heart, lungs, or brain; A, Greenfi
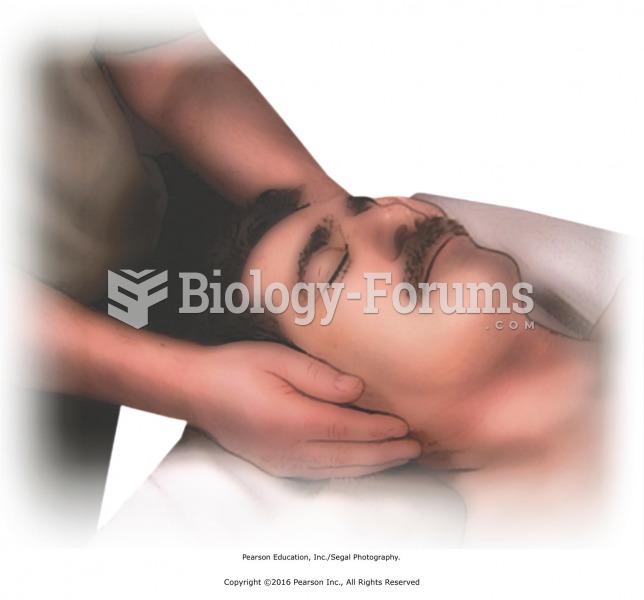
 When using forearm pressure on the top of the shoulder, push with your back foot to increase your ...
When using forearm pressure on the top of the shoulder, push with your back foot to increase your ...
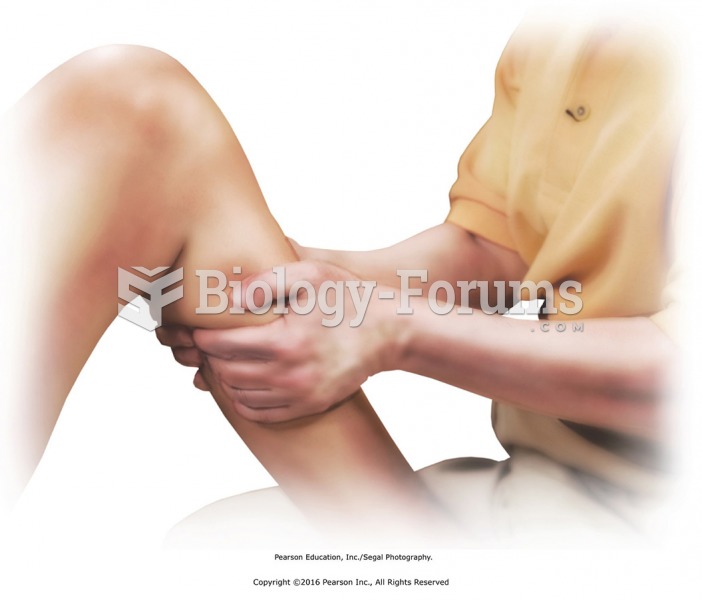 Knead the calf muscles with both hands. Place the foot flat on the table, knee bent. Reach behind ...
Knead the calf muscles with both hands. Place the foot flat on the table, knee bent. Reach behind ...