|
|
|
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
Stevens-Johnson syndrome and Toxic Epidermal Necrolysis syndrome are life-threatening reactions that can result in death. Complications include permanent blindness, dry-eye syndrome, lung damage, photophobia, asthma, chronic obstructive pulmonary disease, permanent loss of nail beds, scarring of mucous membranes, arthritis, and chronic fatigue syndrome. Many patients' pores scar shut, causing them to retain heat.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
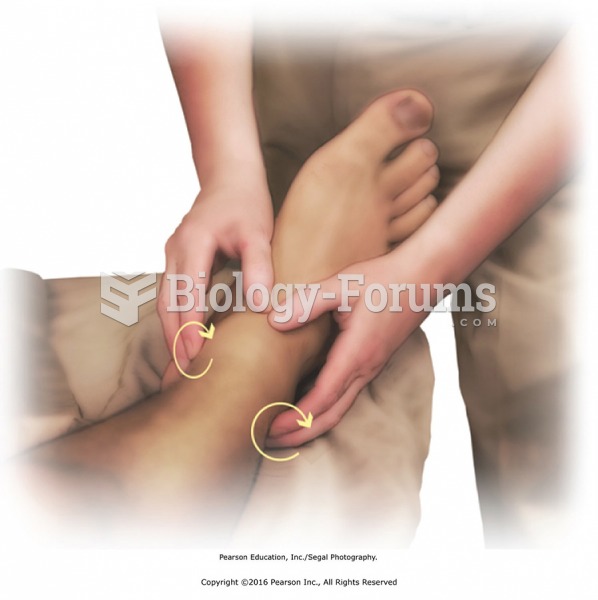 Circular friction around the ankle with the fingertips. Applying pressure with the fingertips, move ...
Circular friction around the ankle with the fingertips. Applying pressure with the fingertips, move ...
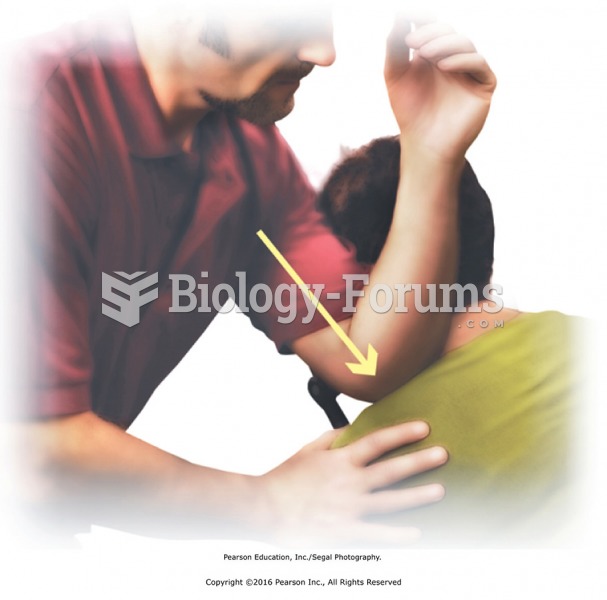 Apply direct pressure to points along the shoulders. Stand to one side and use your forearm to apply ...
Apply direct pressure to points along the shoulders. Stand to one side and use your forearm to apply ...