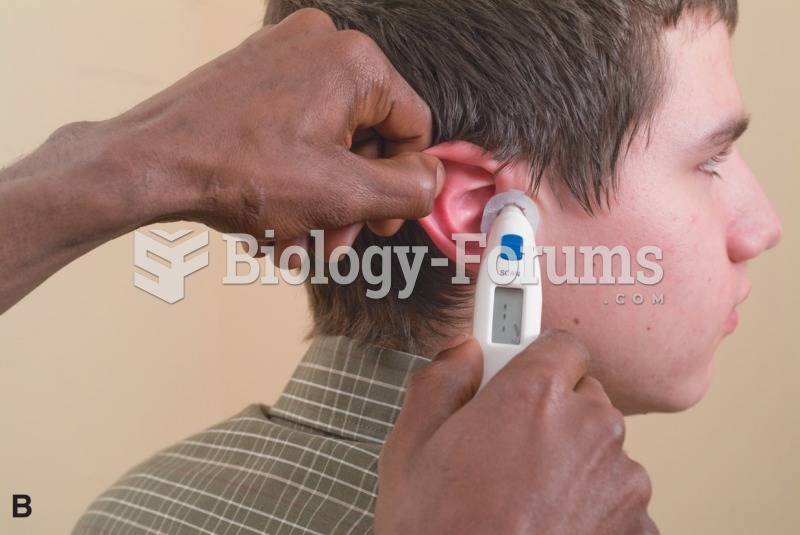
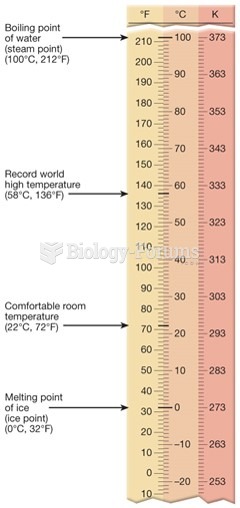
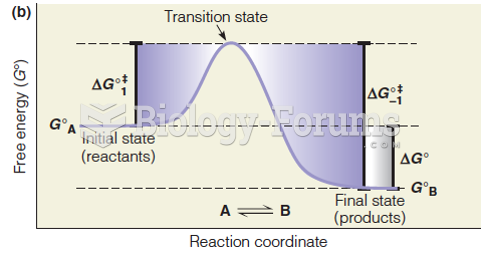
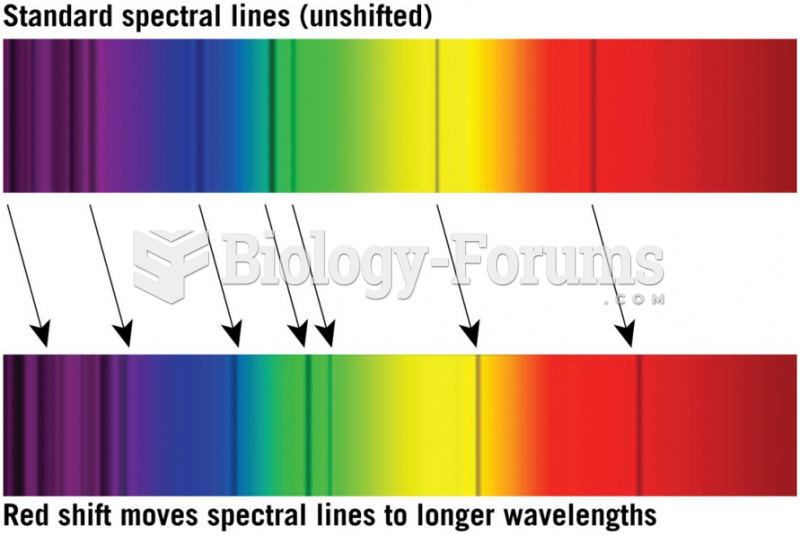
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Did you know?
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.