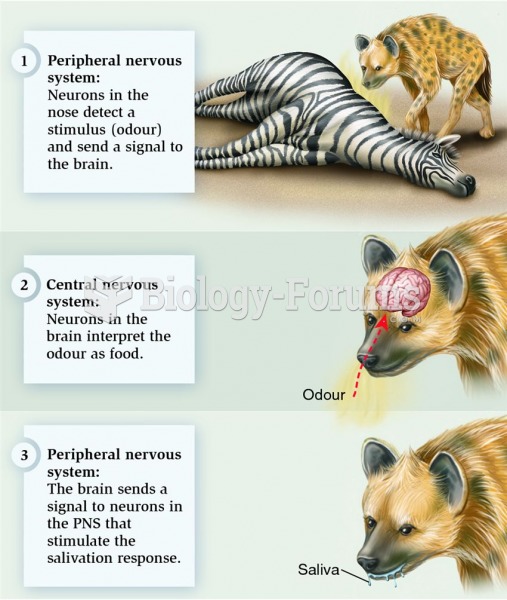
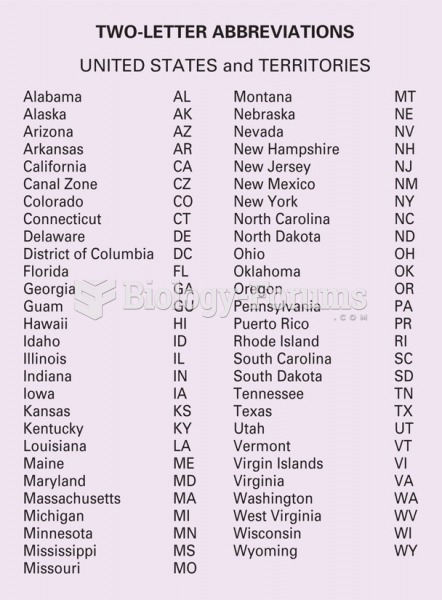
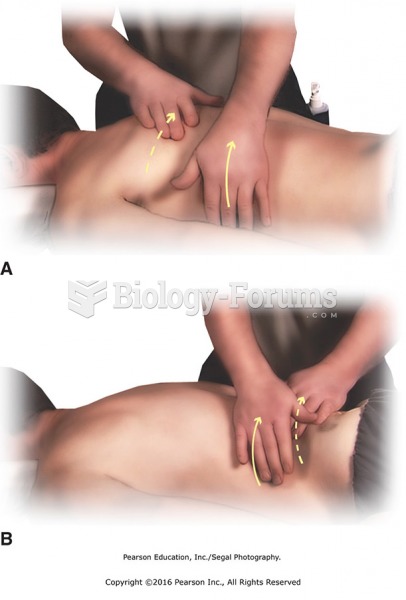
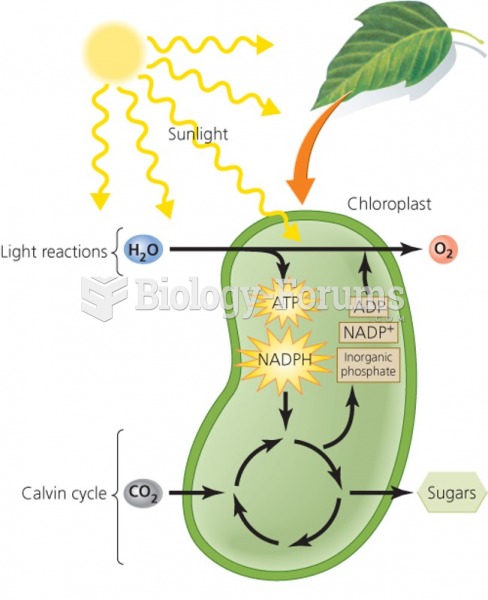
|
|
|
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.