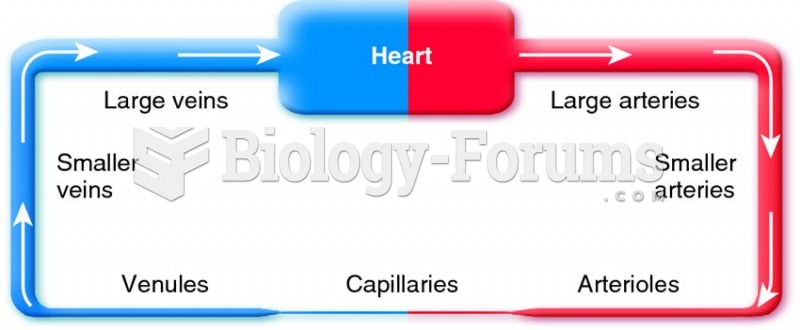
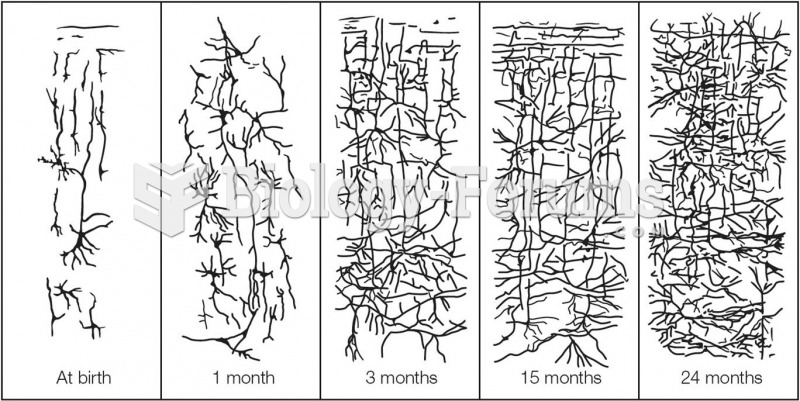
|
|
|
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
In 1864, the first barbiturate (barbituric acid) was synthesized.
It is believed that the Incas used anesthesia. Evidence supports the theory that shamans chewed cocoa leaves and drilled holes into the heads of patients (letting evil spirits escape), spitting into the wounds they made. The mixture of cocaine, saliva, and resin numbed the site enough to allow hours of drilling.
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
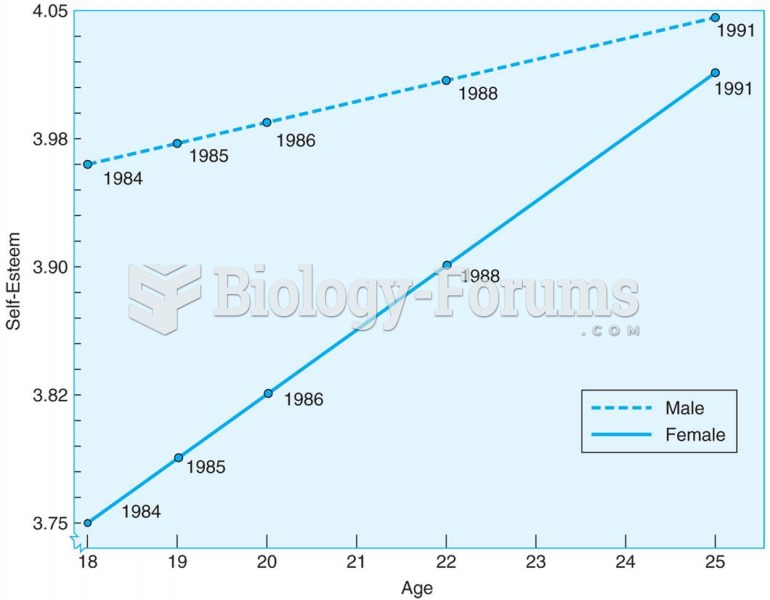 Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s
Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s
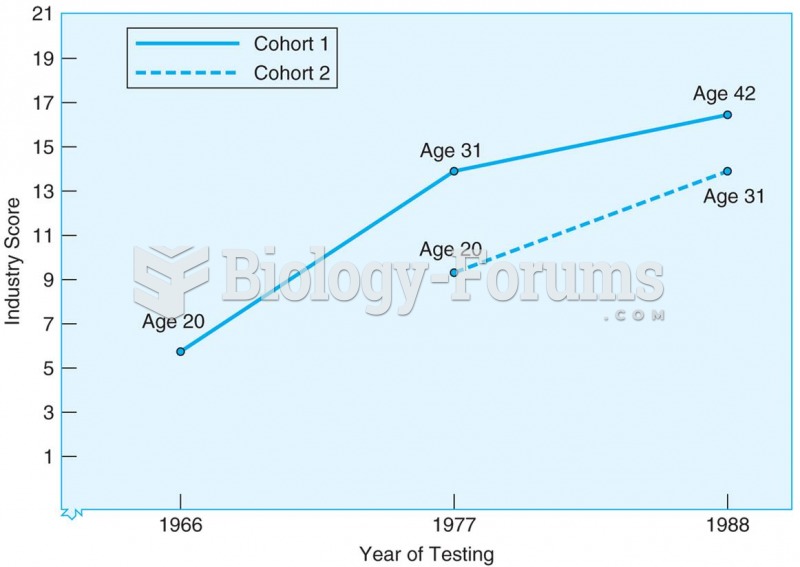 Results from sequential study of two cohorts tested at three ages and at three different points in t
Results from sequential study of two cohorts tested at three ages and at three different points in t