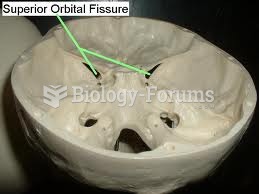
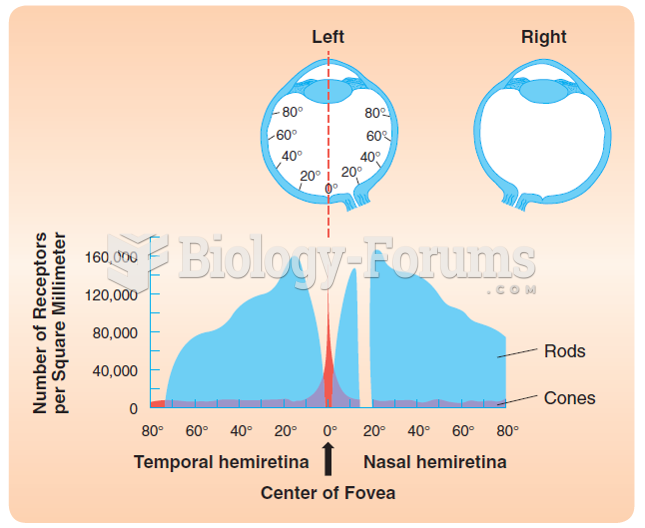
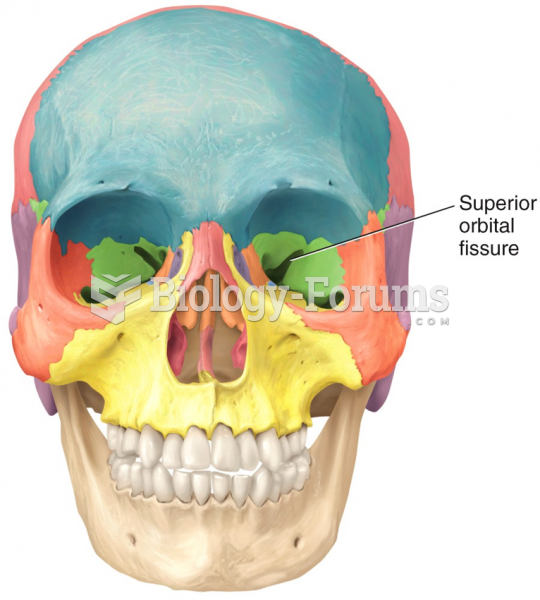
|
|
|
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
 Eighteen-year-olds were the largest age cohort in the first year of the war in both armies. Soldiers
Eighteen-year-olds were the largest age cohort in the first year of the war in both armies. Soldiers
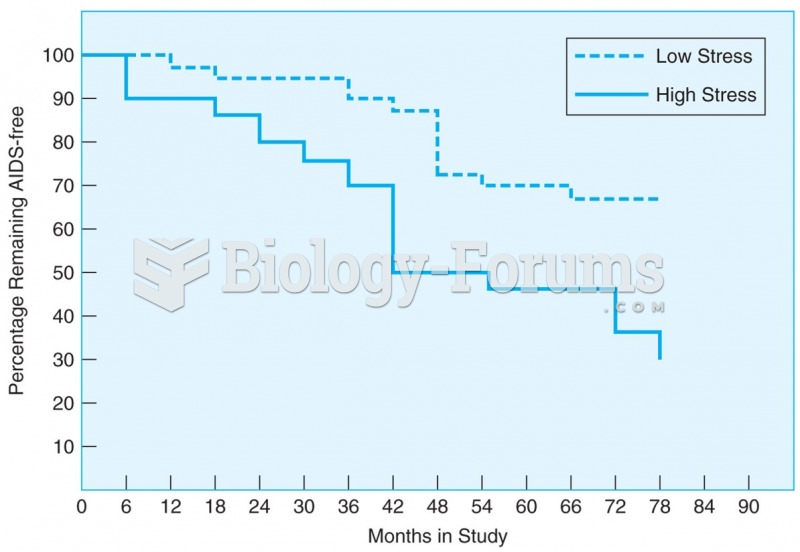 HIV-positive men with a high number of life stressors progress more quickly to AIDS than those with ...
HIV-positive men with a high number of life stressors progress more quickly to AIDS than those with ...