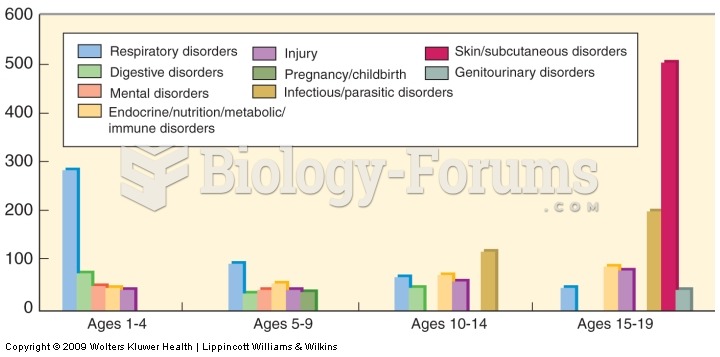
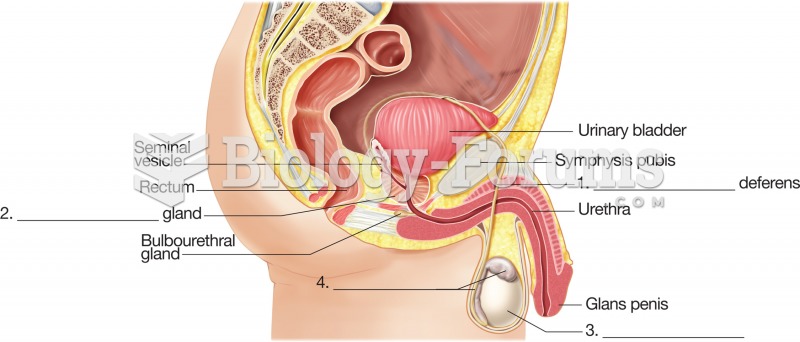
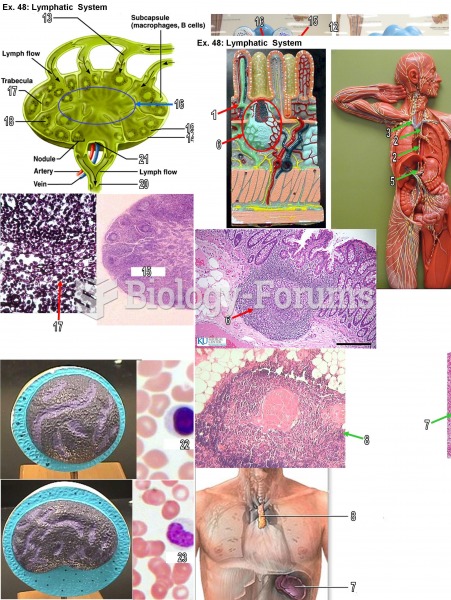
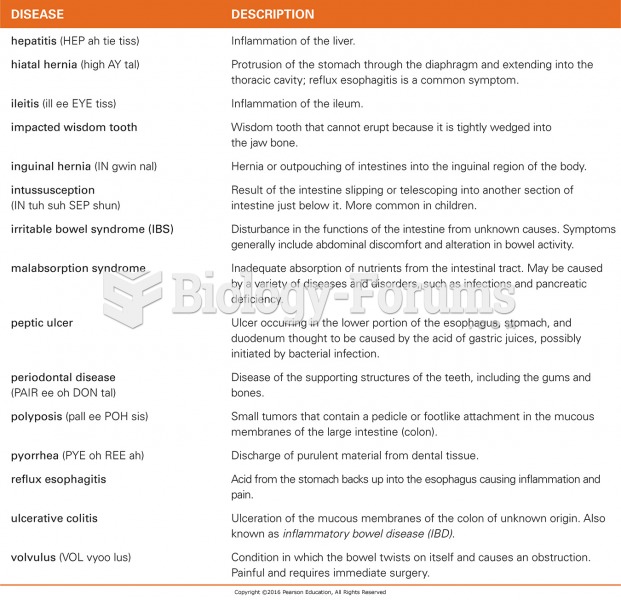
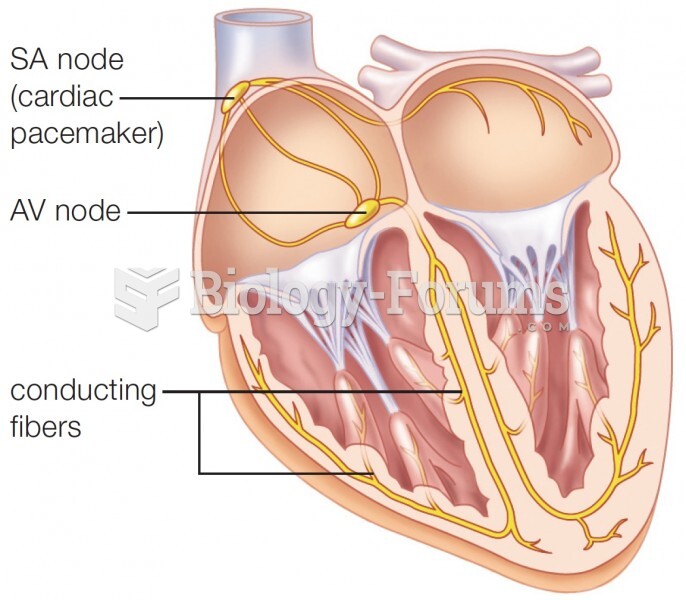
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.