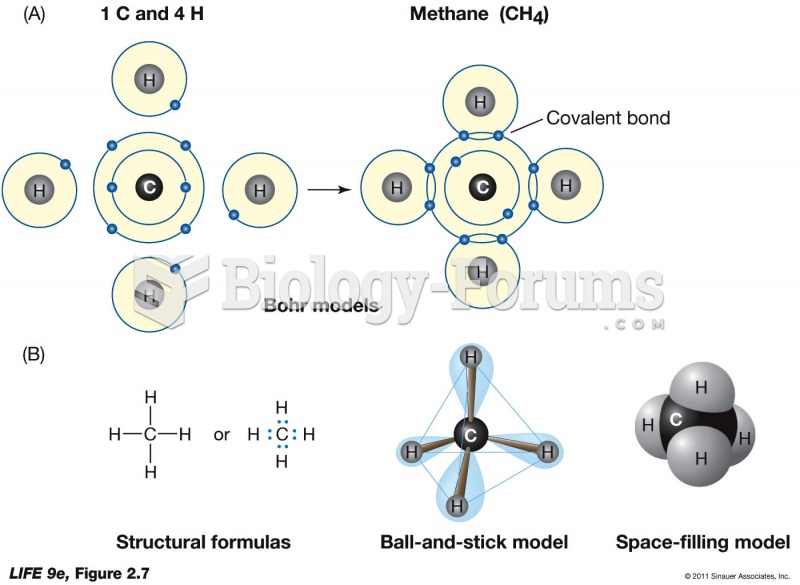
|
|
|
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.
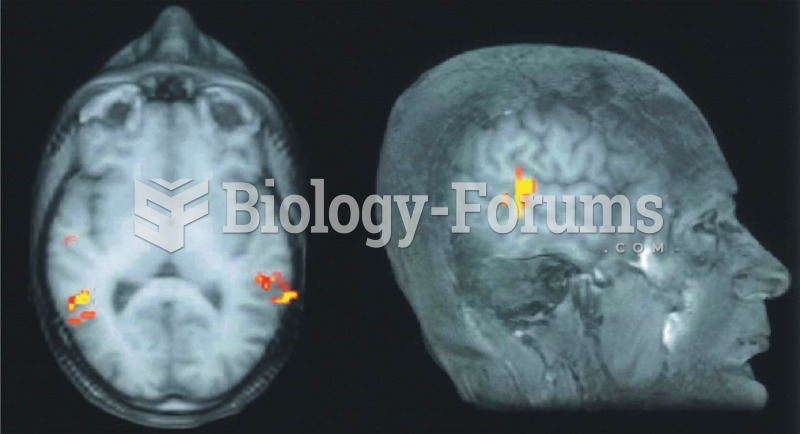
There are immediate benefits of chiropractic adjustments that are visible via magnetic resonance imaging (MRI). It shows that spinal manipulation therapy is effective in decreasing pain and increasing the gaps between the vertebrae, reducing pressure that leads to pain.
The familiar sounds of your heart are made by the heart's valves as they open and close.
Illness; diuretics; laxative abuse; hot weather; exercise; sweating; caffeine; alcoholic beverages; starvation diets; inadequate carbohydrate consumption; and diets high in protein, salt, or fiber can cause people to become dehydrated.
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.