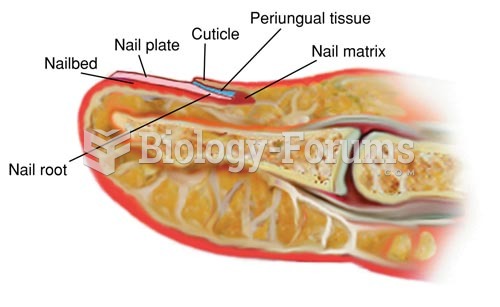
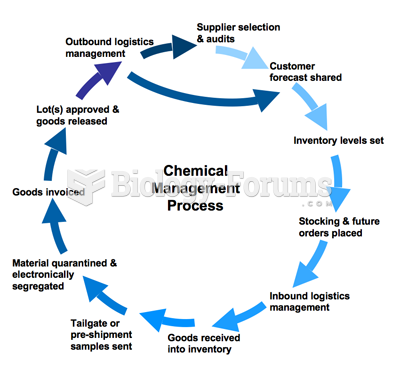
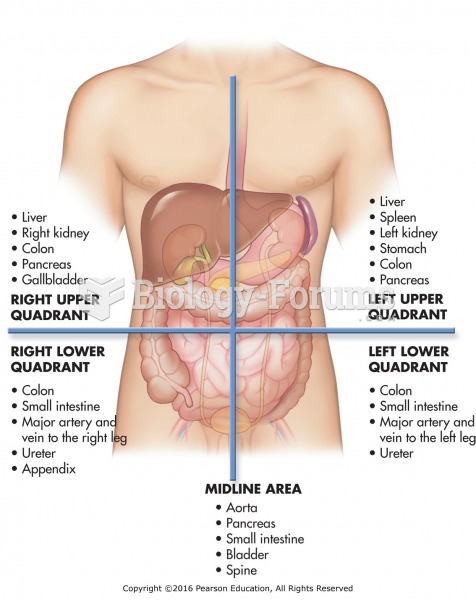
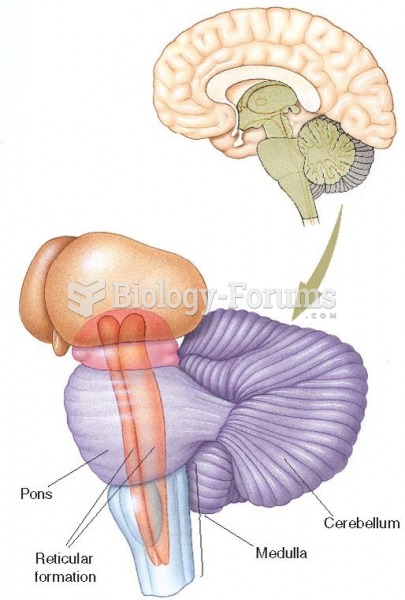
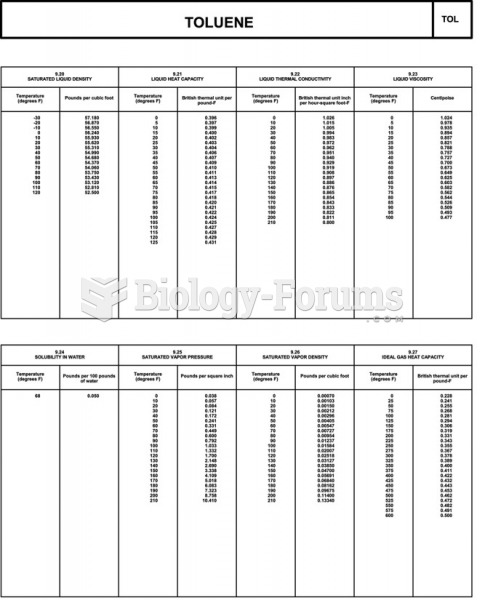
|
|
|
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
There are approximately 3 million unintended pregnancies in the United States each year.
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
There are 20 feet of blood vessels in each square inch of human skin.