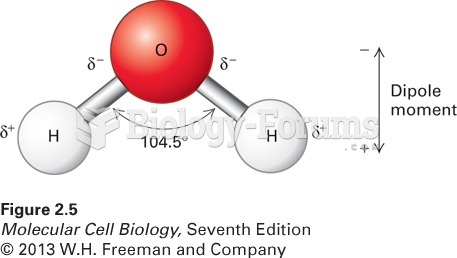
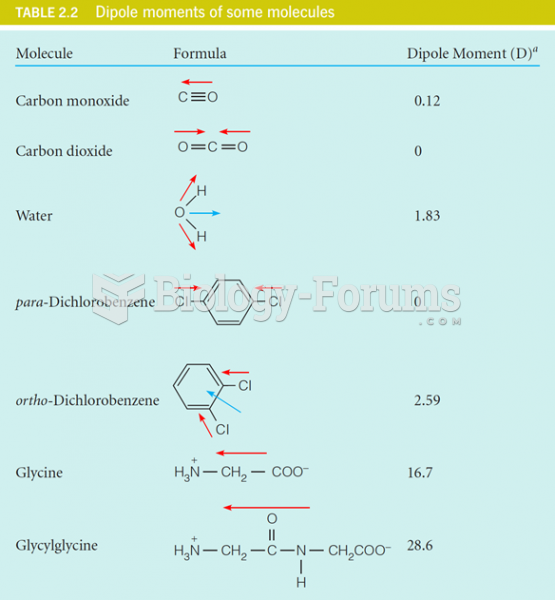
Which of the following exhibits a net dipole moment?
a. SO2
b. CO2
c. O2
d. a and b
e. a, b, and c
Question 2Which of the following does not exhibit a net dipole moment of zero?
a. CO2
b. CH4
c. CCl4
d. H2O
e. SO3
Question 3Elements from the same group
a. have the same number of neutrons.
b. have the same number of valence electrons.
c. usually form the same number of bonds.
d. occur in horizontal rows.
e. b and c
Question 4Which structure could best illustrate the octet rule?
a. He
b. H2
c. SiH4
d. BH3
e. none of these
Question 5An element may form an ion
a. that has a noble gas electron configuration.
b. if the charge is small in relation to its volume.
c. if the element is a metal, metalloid, or nonmetal.
d. a, b, and c
e. b and c
Question 6The noble gases do not readily form chemical bonds because
a. they are gases.
b. they have an even number of protons and electrons.
c. they have a filled outer shell of electrons.
d. their electrons do not allow bonding with other elements.
e. none of these
Question 7Ionic bonding
a. occurs when metals are combined with non metals.
b. involves a sharing of electrons between metal and non metal.
c. occurs when crystals form.
d. occurs when the inner core electrons of the metal are transferred to the non metal.
e. none of these
Question 8Covalent bonding
a. involves a transfer of electrons from one atom to another.
b. occurs when atoms share all their valence electrons.
c. occurs when unpaired valence electrons are shared between atoms.
d. usually produces very polar compounds.
e. none of these
Question 9Which compound contains ionic bonds?
a. NI3
b. CH3COCl
c. NaHCO3
d. NH3
e. SiO2
Question 10Which compound(s) is (are) ionic?
a. BF3
b. NaNH2
c. Li2CO3
d. a and b
e. b and c
Question 11The dots in Lewis structures represent
a. all electrons.
b. valence electrons.
c. the number of protons.
d. the charge.
e. none of the above
Question 12All atoms of a given element have the same
a. number of electrons.
b. number of neutrons.
c. number of protons.
d. mass.
e. number of protons and electrons.
Question 13Valence electrons refer to those
a. in the nearest shell.
b. which are negative.
c. of lowest energy.
d. in the outermost shell.
e. that are electronegative.
Question 14The electrons are located
a. in the nucleus.
b. in orbitals.
c. in perfectly circular orbits.
d. in and around the nucleus.
e. in nodes.
Question 15The atomic nucleus
a. is positively charged.
b. has no charge (neutral) because of the neutrons.
c. is neutral because of the electrons.
d. is negatively charged.
e. none of the above
Question 16The nucleus of an atom consists of
a. protons.
b. protons and neutrons.
c. electrons, proton, and neutrons.
d. protons and electrons.
e. neutrons and electrons.
Question 17The basic chemical building block is the
a. proton.
b. ion.
c. atom.
d. electron.
e. neutron.
Question 18Modern organic chemistry
a. is the study of carbon-containing compounds.
b. is the study of compounds from living organisms.
c. deals exclusively with chemicals that are obtainable from natural sources.
d. a, b, and c
e. a and c
Question 19A compound with the formula C5H7NO2 has these peaks in its IR spectrum: 3000-2850 cm1; 2250 cm1;
1740 cm1 (intense), and 1200 cm1 (intense). Explain which functional group is present in this compound and
show a possible structure for it.
Question 20A compound with the formula C4H10O has these peaks in its IR spectrum: 3300 cm1 (broad); 3000-
2850 cm1; 1050 cm1(intense). Explain which functional group is present in this compound and show a
possible structure for it.
Question 21How would you use infrared spectroscopy to distinguish 1-propanol and propanoic acid?