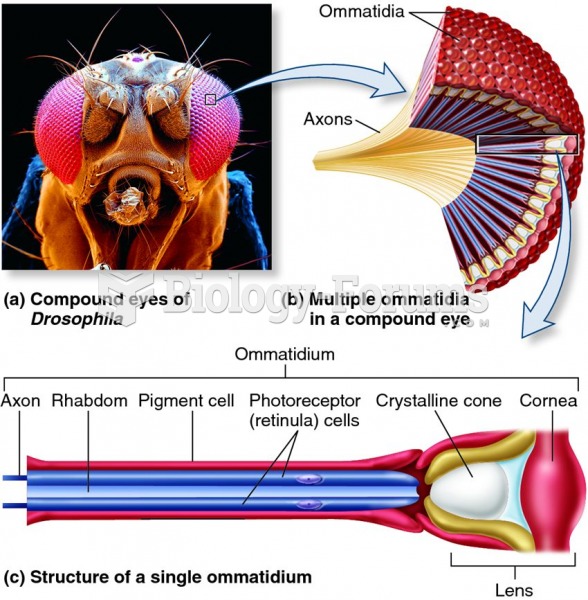
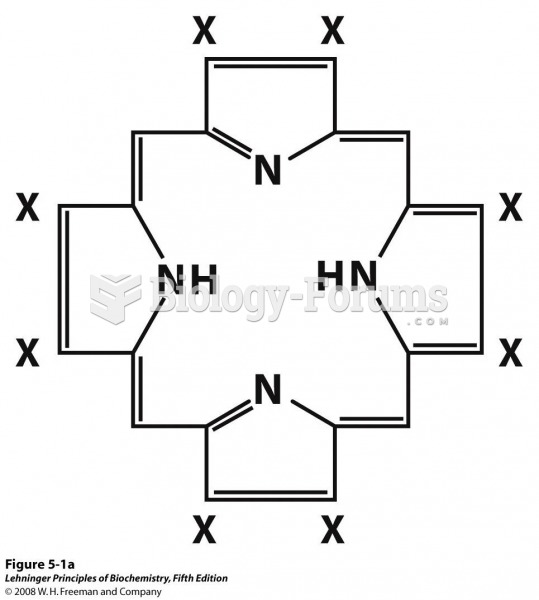
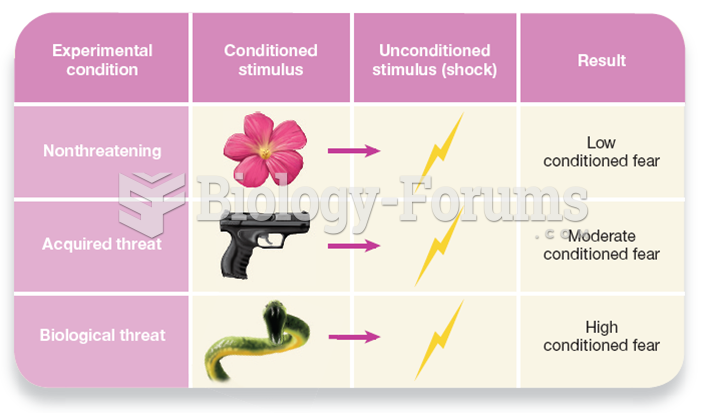
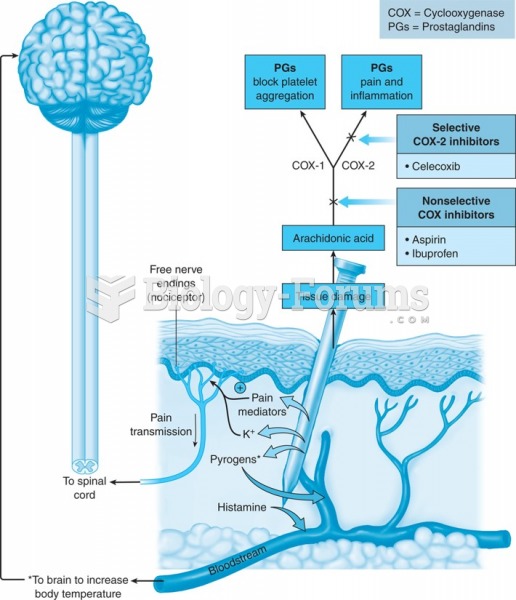
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
Did you know?
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.