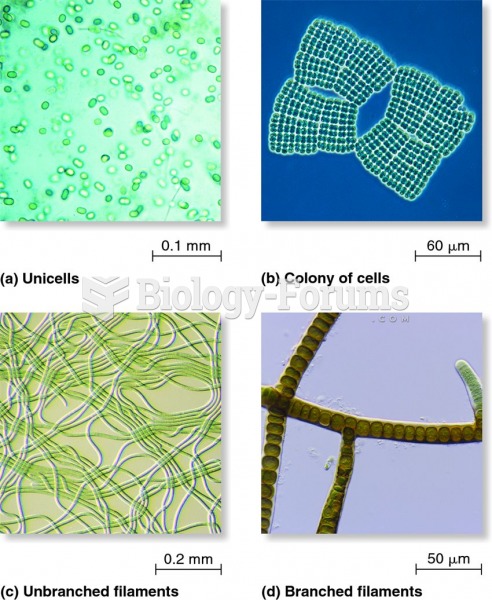
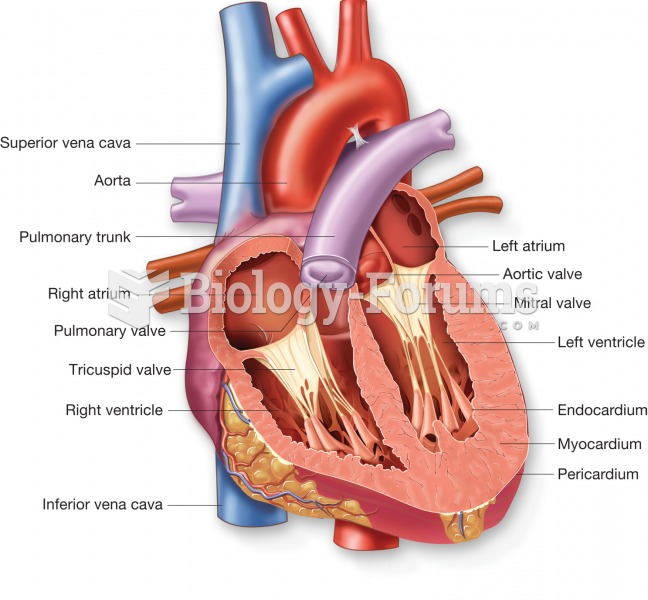
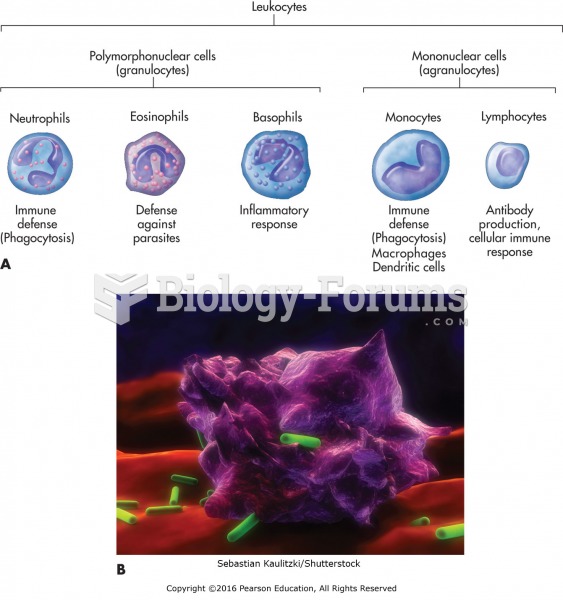
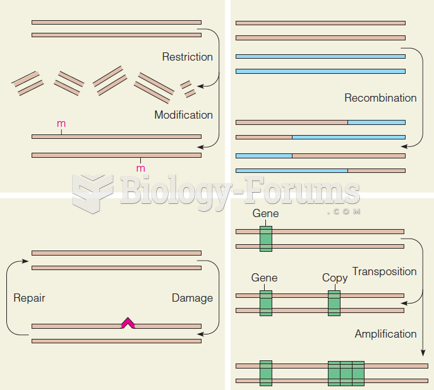
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Automated pill dispensing systems have alarms to alert patients when the correct dosing time has arrived. Most systems work with many varieties of medications, so patients who are taking a variety of drugs can still be in control of their dose regimen.
Did you know?
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.