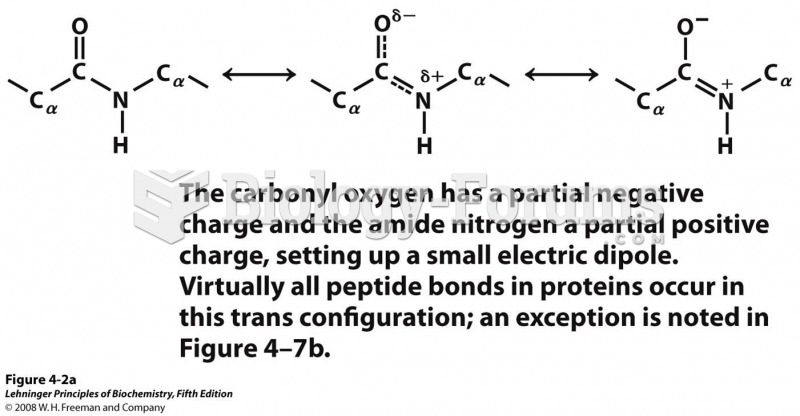
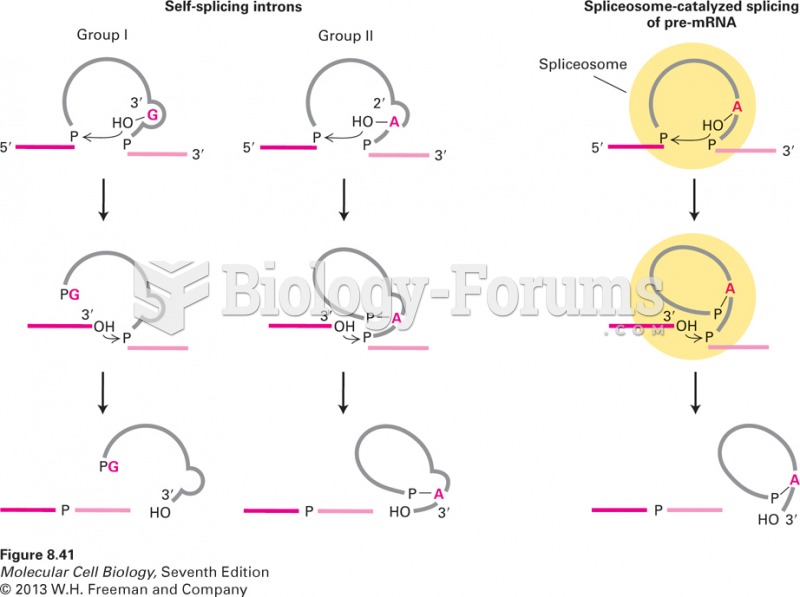
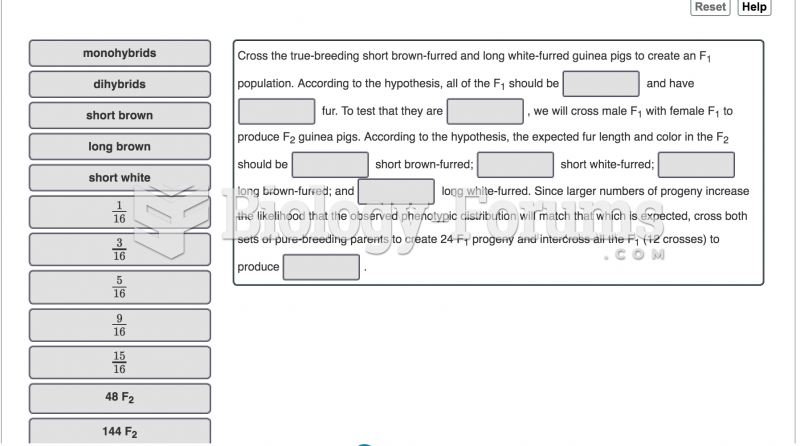
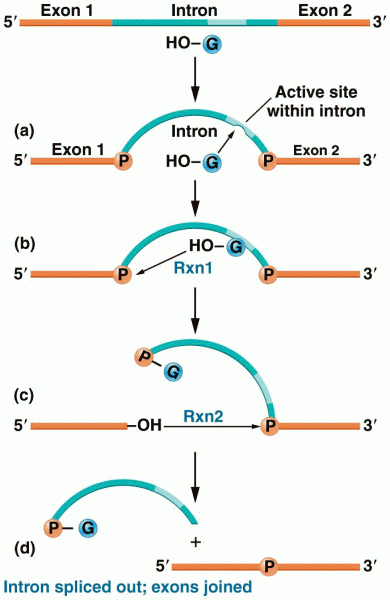
|
|
|
The horizontal fraction bar was introduced by the Arabs.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).