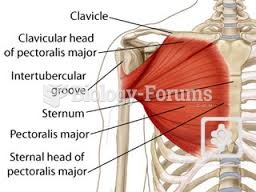
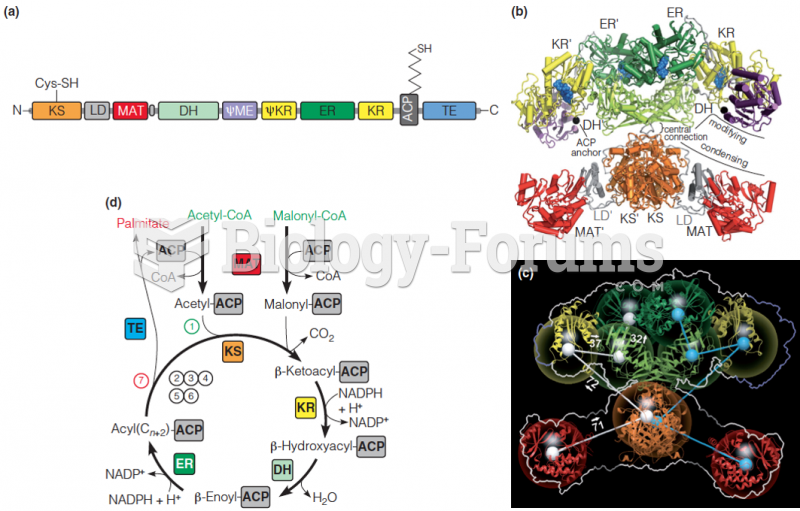
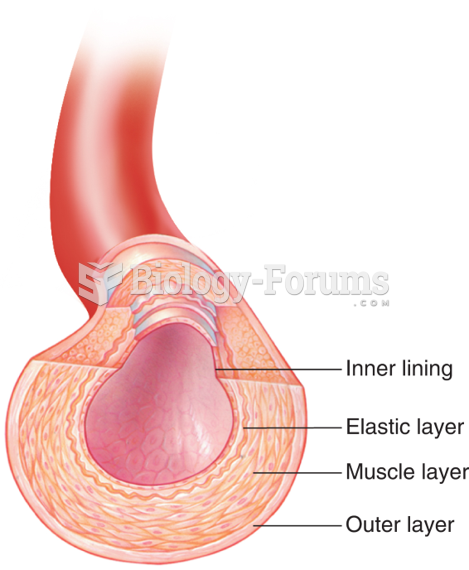
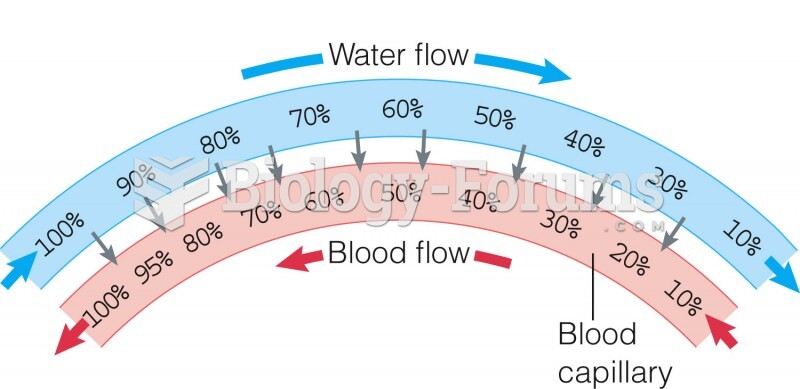
Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 2
Question 2Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 3
Question 3Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 4
Question 4Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 5
Question 5Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 6
Question 6Instructions:
Draw the structure of the major organic products(s) for each of the following reactions. Indicate the stereochemistry for each reaction when appropriate.
 Question 7
Question 7Consider the reactions below to answer the following questions.

Halving the concentration of hydroxide in these reactions:
a.
causes the reaction mechanism to change
b.
halves the rate of reaction
c.
has no effect on the rate of reaction
d.
doubles the rate of reaction
Question 8Consider the reactions below to answer the following questions.

The mechanism for these reactions is:
a.
S
N1
b.
S
N2
c.
E1
d.
E2
Question 9Consider the reactions below to answer the following questions.

Which reaction would be predicted to be faster? Explain.
Question 10Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers).
Question 11Consider the following reaction:

a)
Draw the product of the reaction. How would you categorize this process mechanistically?
b)
Which of the following spectral methods could be used to follow the progress of the reaction?
a. NMR spectroscopy
b. Mass spectrometry
c. Infrared spectroscopy
d. UV spectroscopy
e. all of these
f. a, b, and c only
Question 12Predict the product of the following reaction:
 Question 13
Question 13Which of the following represents the transition state of the S
N2 reaction between methyl iodide and ammonia?
a.

b.

c.

d.
 Question 14
Question 14What is the IUPAC name of the following compound?

a.
(
R)-2-bromo-2-vinylpentane
b.
(
S)-2-bromo-2-vinylpentane
c.
(
S)-3-bromo-3-propylbut-1-ene
d.
(
R)-3-bromo-3-methylhex-1-ene
Question 15Consider the following compound:

a)
What is the IUPAC name of the compound?
a. (
R)-1-chloro-3-methyl-2-cyclohexene
b. (
S)-1-chloro-3-methyl-2-cyclohexene
c. (
R)-3-chloro-1-methylcyclohexene
d. (
S)
-3-chloro-1-methylcyclohexene
b)
How could this compound be used to produce a conjugated diene?
a. substitution
b. elimination
c. allylic free radical formation
d. either b or c
Question 16What is the rate law for the E2 reaction of an alkyl halide (RX) with sodium ethoxide (NaOEt) in ethanol solvent (EtOH)?
a.
rate = kRX
b.
rate = kRX
2 c.
rate = kRXNa
+ d.
rate = kRXOEt
- e.
rate = kOEt
-Question 17Which mechanism is favored by the reaction of a secondary alkyl bromide with potassium
t-butoxide?
a.
S
N1
b.
S
N2
c.
E1
d.
E1
CB e.
E2