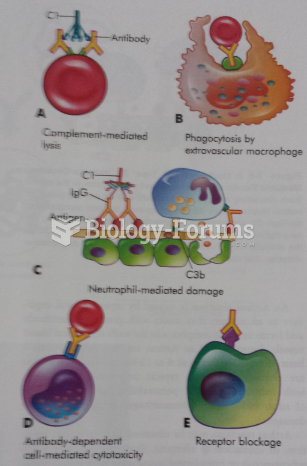
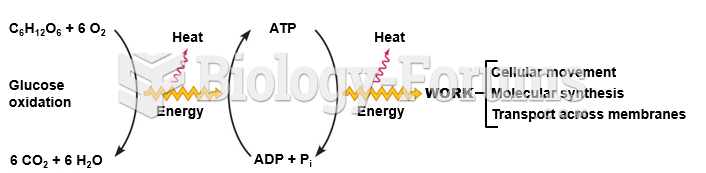
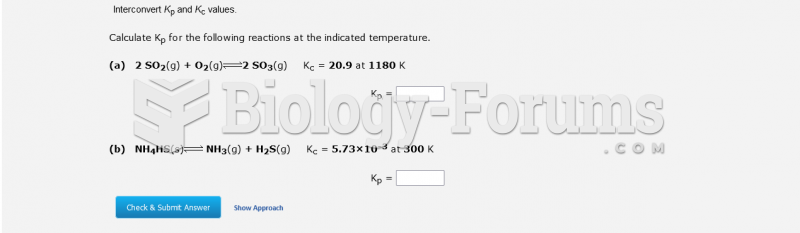
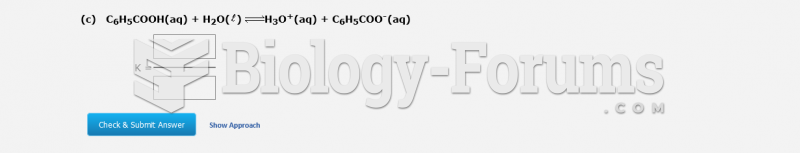
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Did you know?
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Did you know?
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.