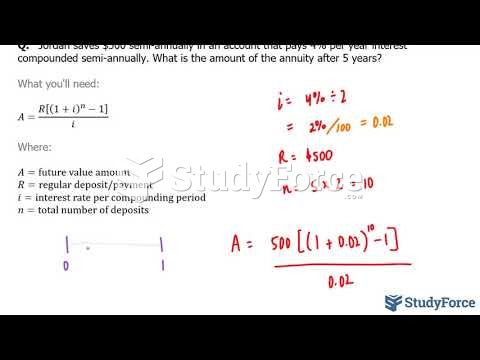
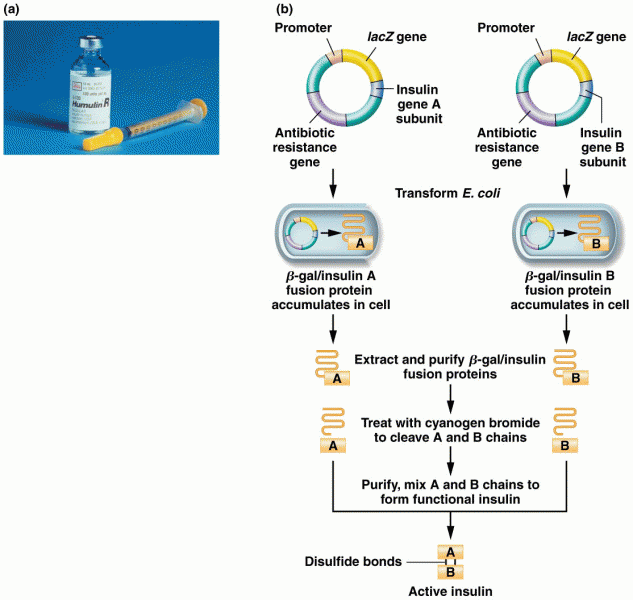
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.