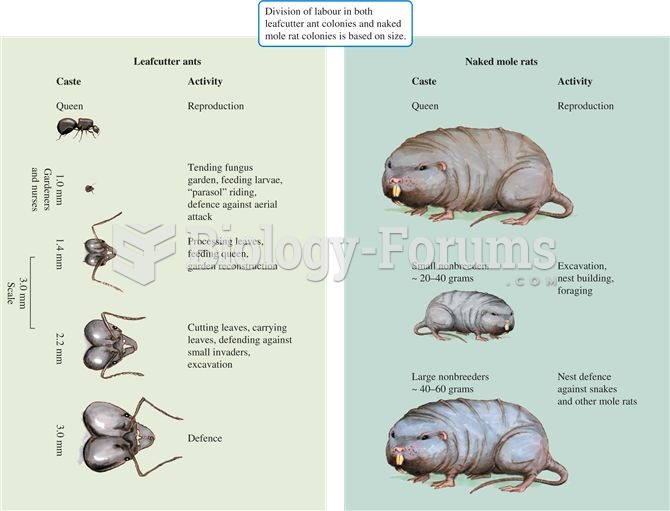
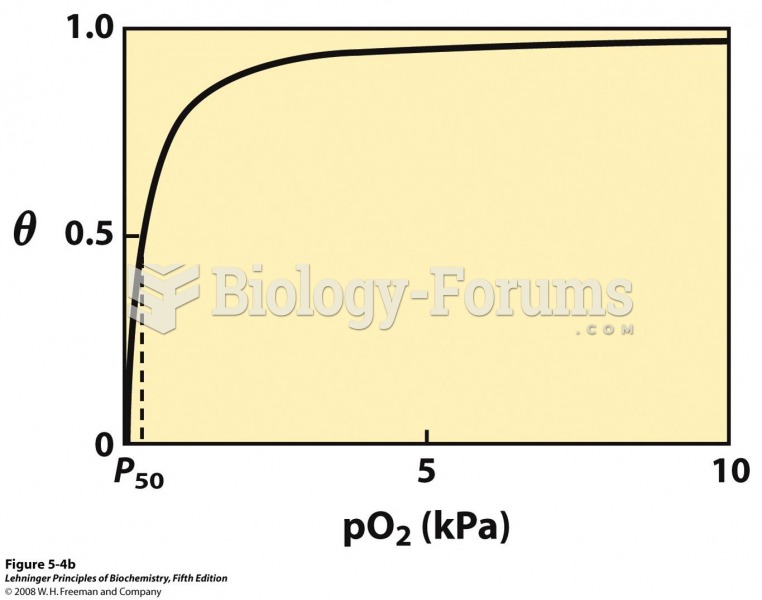
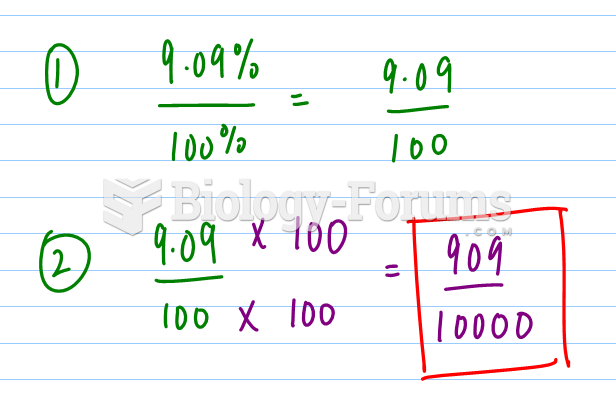
|
|
|
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Asthma cases in Americans are about 75% higher today than they were in 1980.
Over time, chronic hepatitis B virus and hepatitis C virus infections can progress to advanced liver disease, liver failure, and hepatocellular carcinoma. Unlike other forms, more than 80% of hepatitis C infections become chronic and lead to liver disease. When combined with hepatitis B, hepatitis C now accounts for 75% percent of all cases of liver disease around the world. Liver failure caused by hepatitis C is now leading cause of liver transplants in the United States.
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.