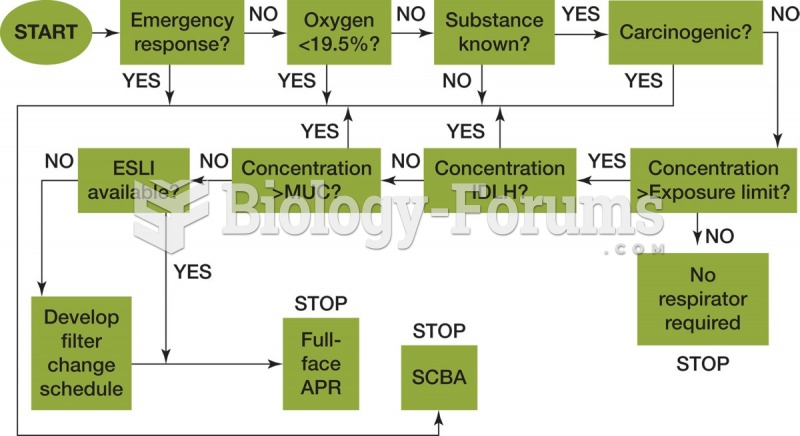
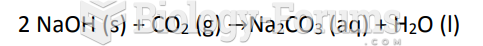|
|
|
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
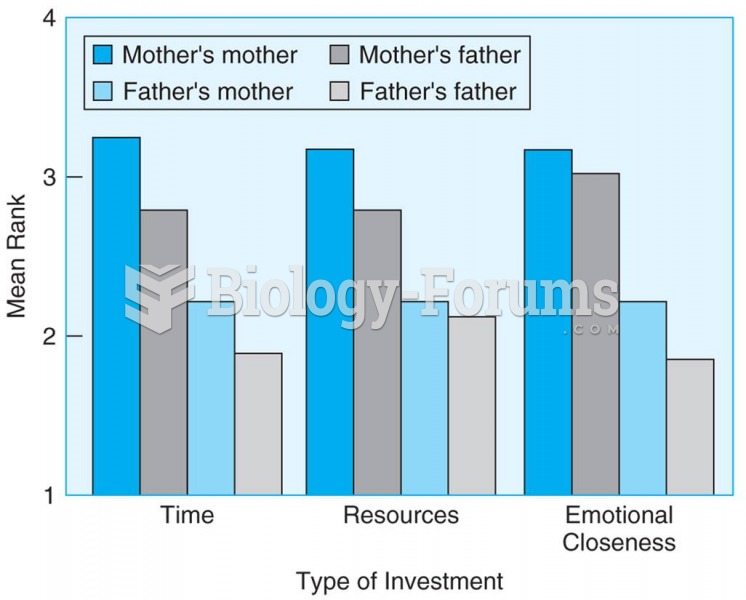 Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...
Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...
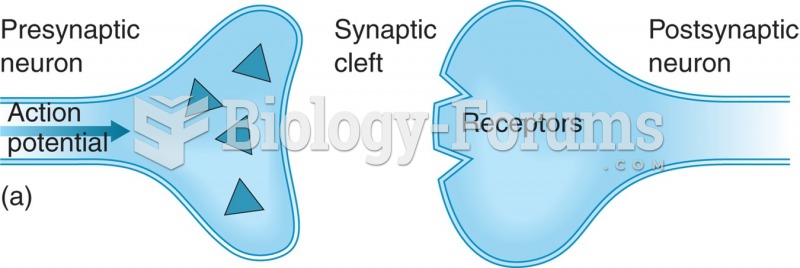 Synaptic transmission. (a) Action potential reaches synapse; (b) neurotransmitter released synaptic ...
Synaptic transmission. (a) Action potential reaches synapse; (b) neurotransmitter released synaptic ...