|
|
|
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
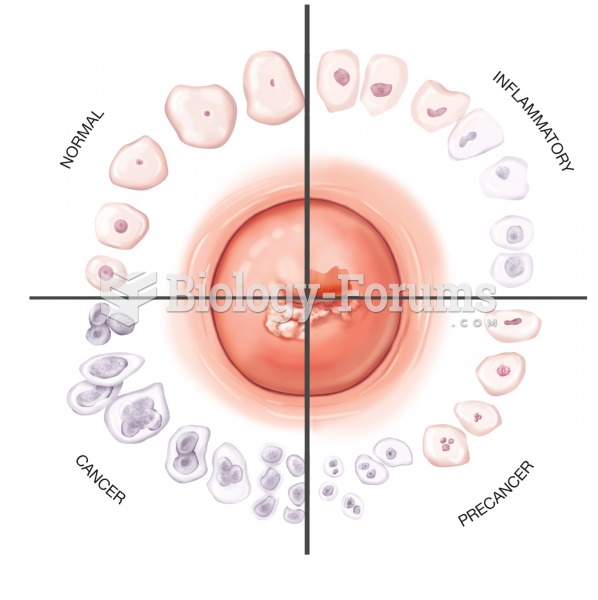
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.