This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
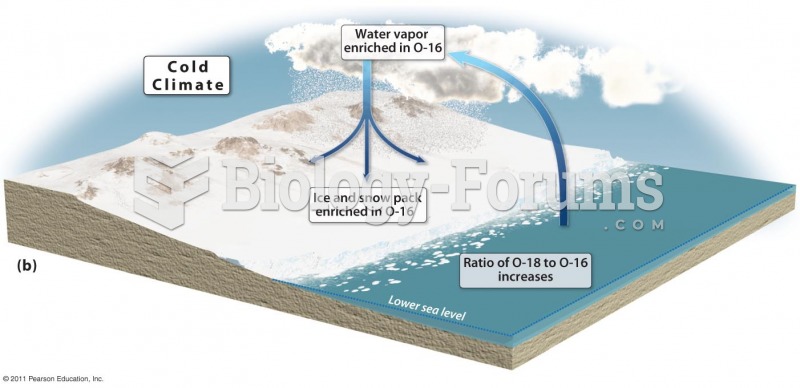
The senior population grows every year. Seniors older than 65 years of age now comprise more than 13% of the total population. However, women outlive men. In the 85-and-over age group, there are only 45 men to every 100 women.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Did you know?
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.