|
|
|
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
The most dangerous mercury compound, dimethyl mercury, is so toxic that even a few microliters spilled on the skin can cause death. Mercury has been shown to accumulate in higher amounts in the following types of fish than other types: swordfish, shark, mackerel, tilefish, crab, and tuna.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
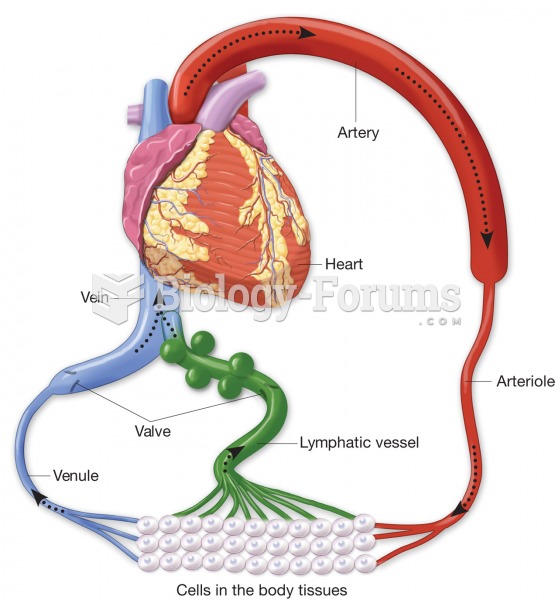 Lymphatic vessels (green) pick up excess tissue fluid, purify it in lymph nodes, and return it to th
Lymphatic vessels (green) pick up excess tissue fluid, purify it in lymph nodes, and return it to th
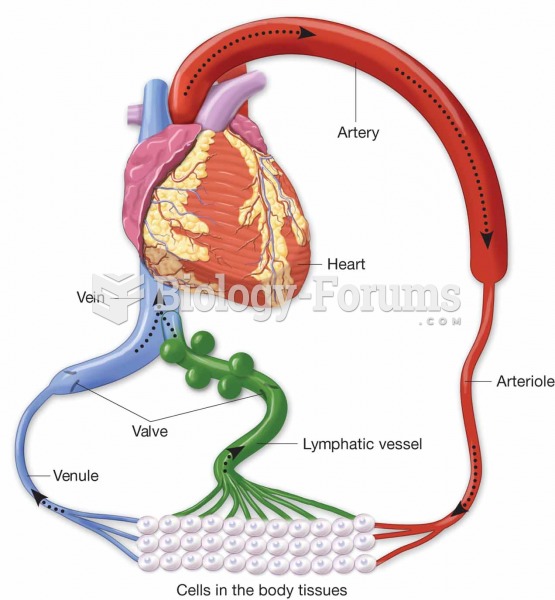 Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t
Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t