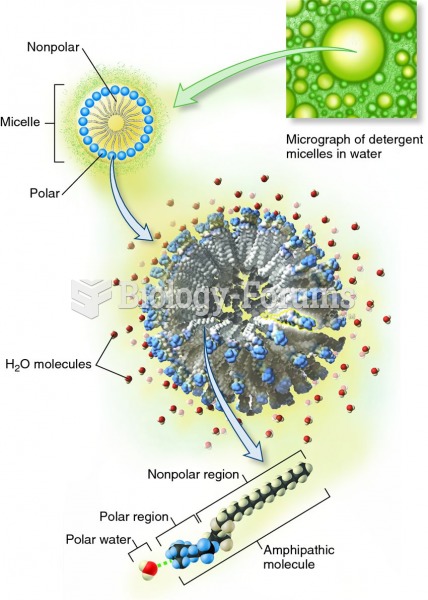
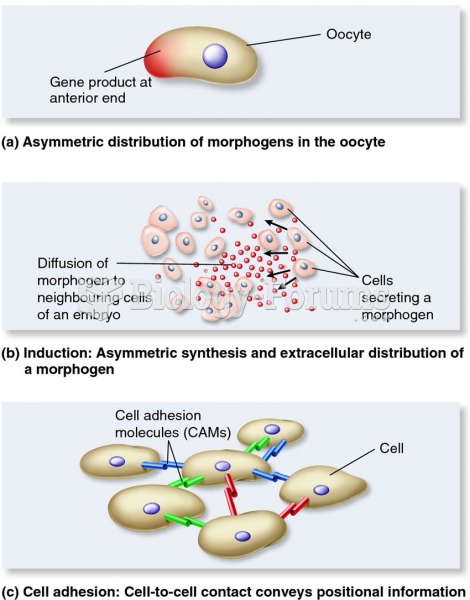
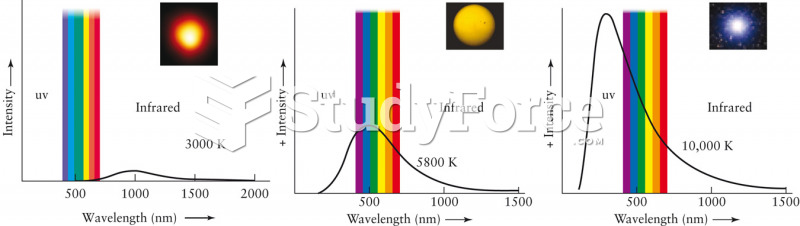
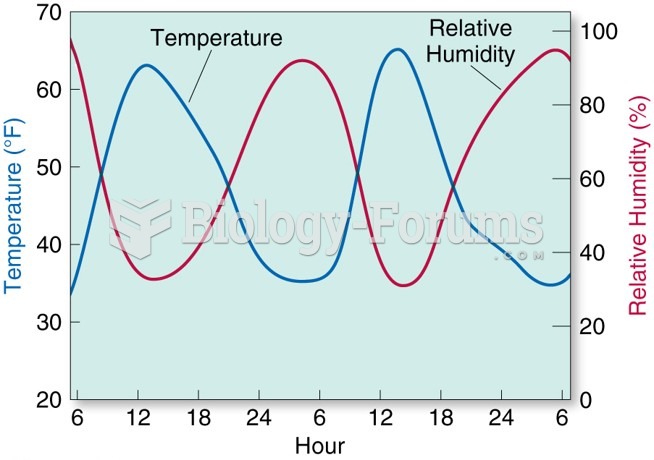
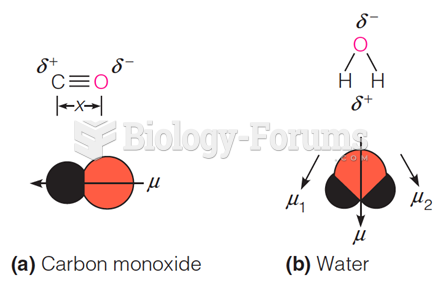
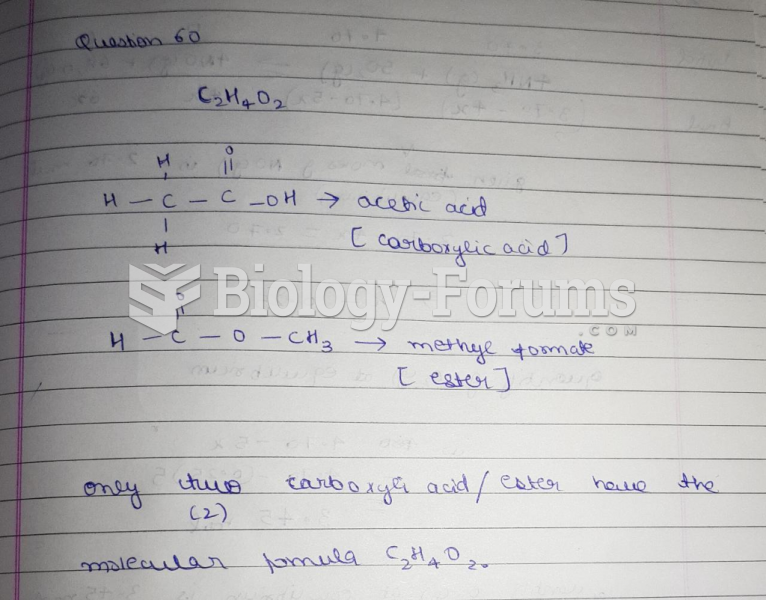
|
|
|
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.
The use of salicylates dates back 2,500 years to Hippocrates’s recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.