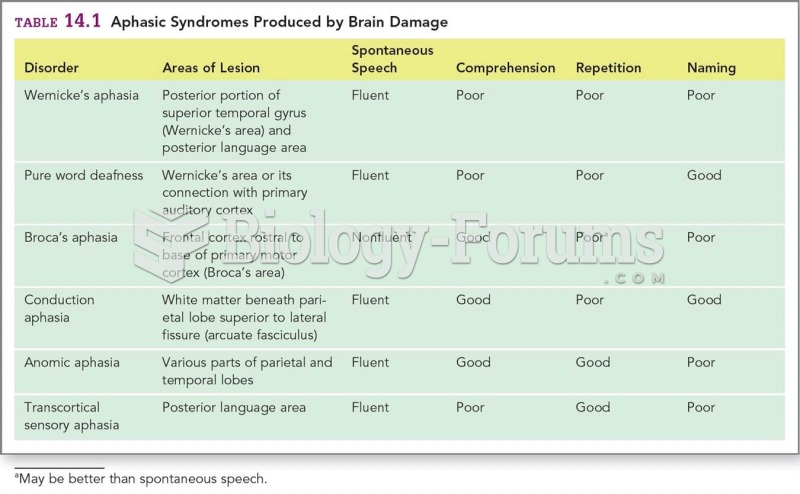
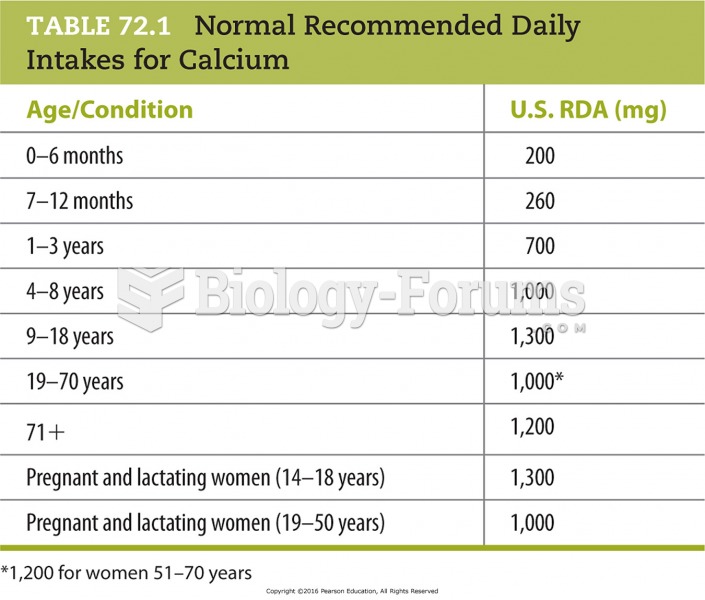
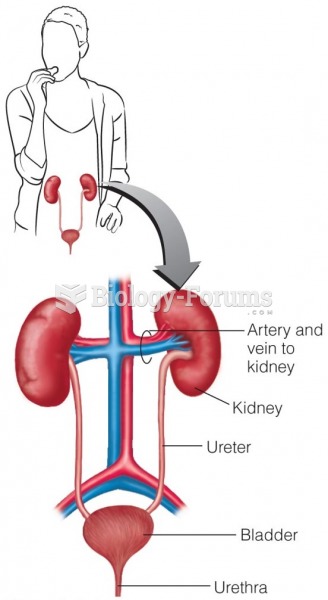
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.