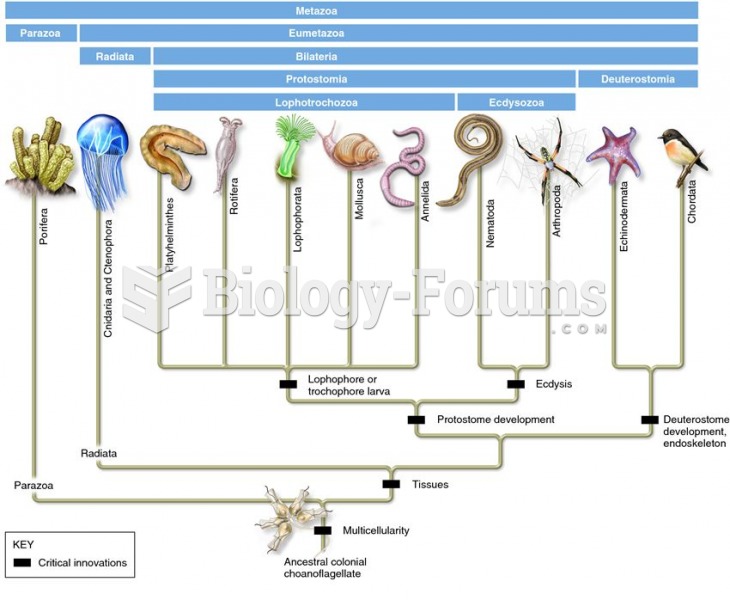
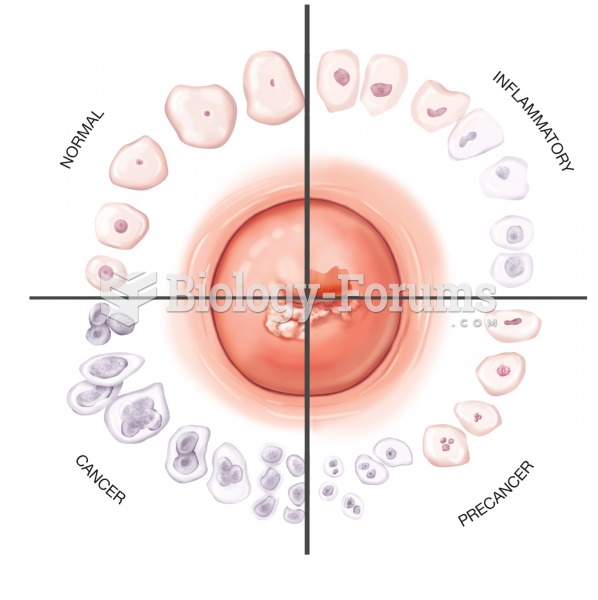
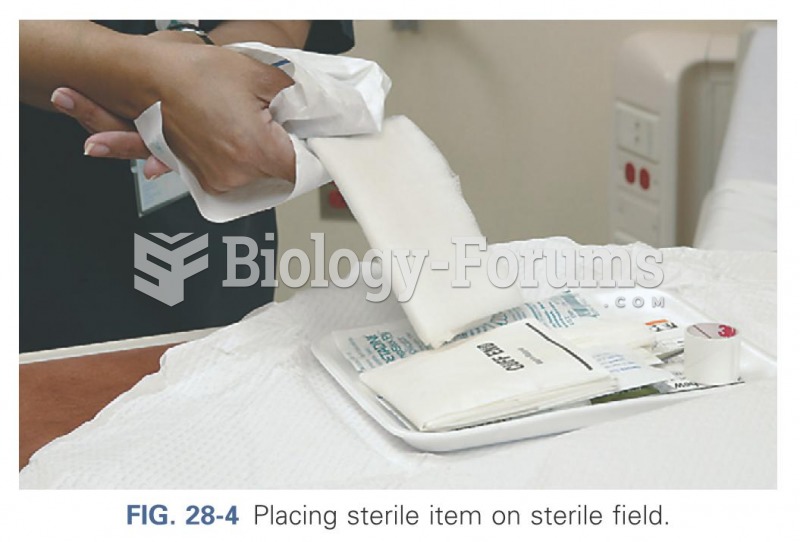
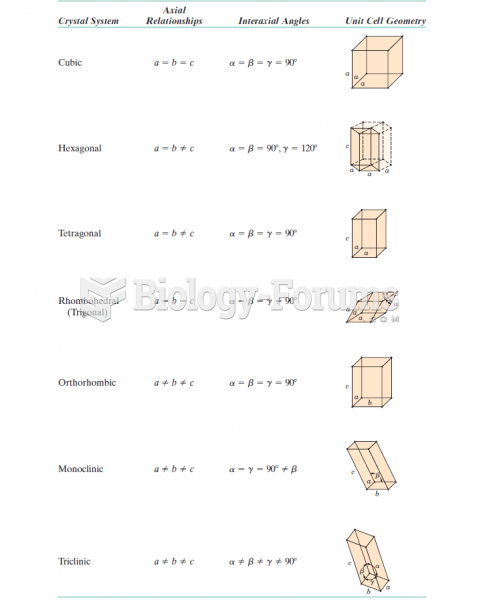
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are immediate benefits of chiropractic adjustments that are visible via magnetic resonance imaging (MRI). It shows that spinal manipulation therapy is effective in decreasing pain and increasing the gaps between the vertebrae, reducing pressure that leads to pain.
Did you know?
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
The FDA recognizes 118 routes of administration.