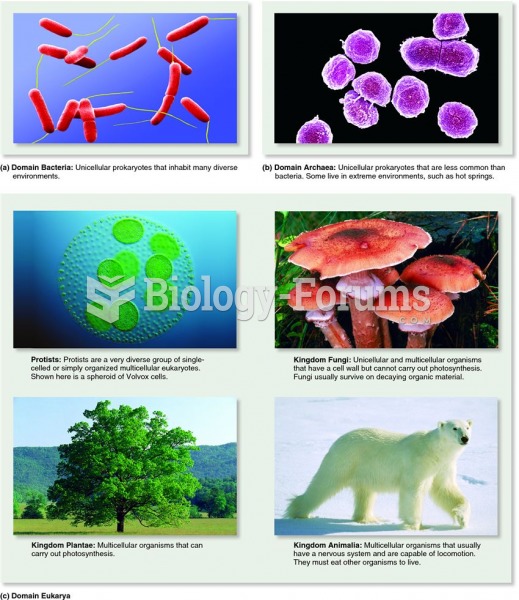
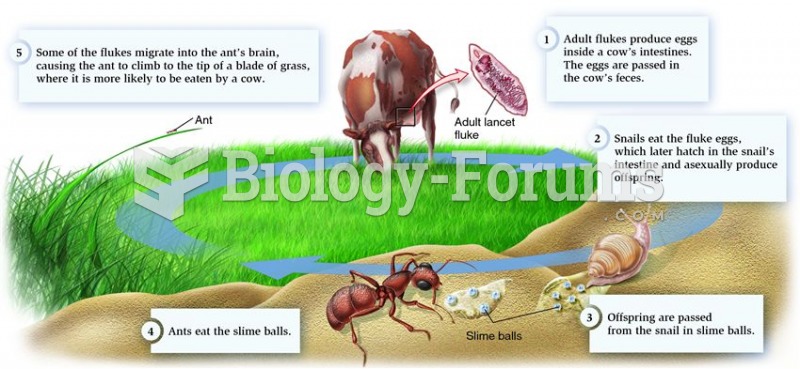
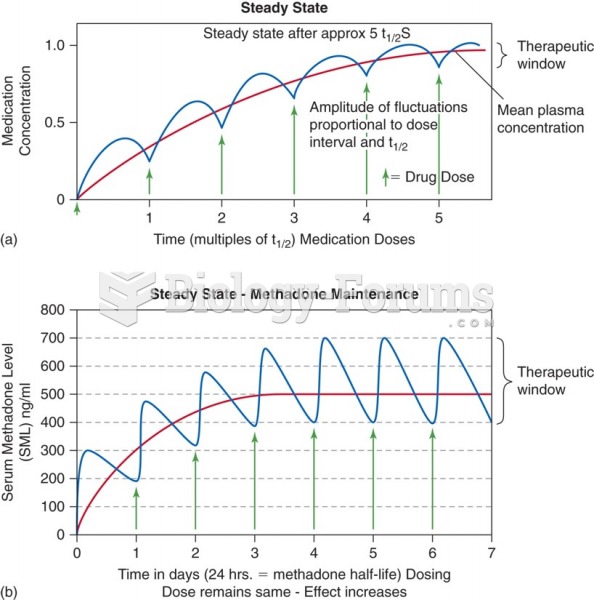
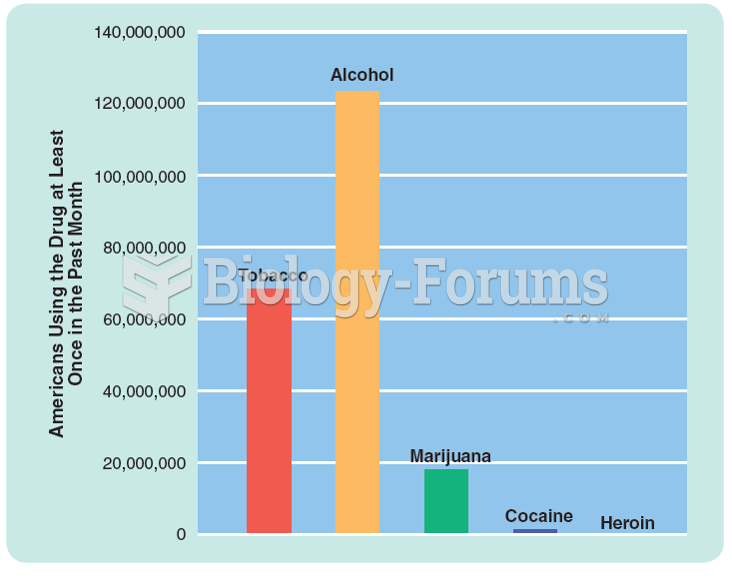
|
|
|
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.