This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
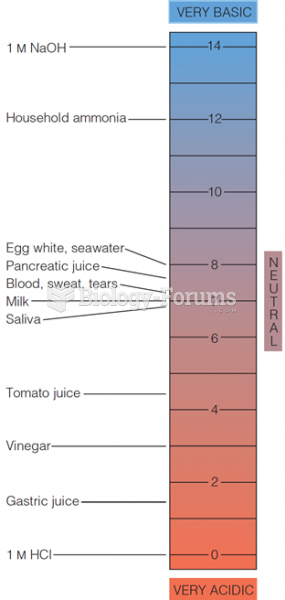
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.